Abstract
Objectives
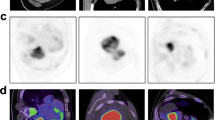
CT-guided biopsy of indeterminate lung lesions sometimes provides insufficient histological results due to tumor necrosis. Functional and metabolic methods such as DWI-MR and PET-CT may help by directing sample collection to a lesion area of greater biological representativeness. The objective is to evaluate the histopathological results based on findings on ADC and SUV levels in lung lesions suspected for primary cancer.
Methods
Tissue samples were evaluated after undergoing biopsies guided by either DWI-MR or PET-CT findings. In each patient, sample collection from two lesion areas was guided by local ADC and SUV. Values were used to define areas of low vs. high suspicion for cancer.
Results
Patients who underwent DWI-MR had median lesion size of 78.0 mm. Areas of higher suspicion (HSA) had a median ADC of 1.1 × 10-3 mm2/s, while areas of lower suspicion (LSA) had median ADC of 1.8 × 10-3 mm2/s (p = 0.0001). All HSA samples and 71.43% of LSA samples were positive for cancer (p = 0.0184). Patients who performed PET-CT had median lesion size of 61.0 mm. Median SUV was 7.1 for HSA and 3.9 for LSA (p = 0.0002). Positivity for cancer was observed in 76.9% of samples for both HSA and LSA (p = 0.0522).
Conclusion
Use of DWI-MR and PET-CT showed that tumors are functional and metabolically heterogeneous and that this heterogeneity has implications for histopathological diagnosis.
Key Points
• Lung cancer is heterogeneous regarding functional and metabolic imaging.
• Tumor heterogeneity may have implications in histopathological diagnosis.
• Intralesional lower levels of ADC target highly suspected areas with a significant improvement in lung cancer diagnosis.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CT:
-
Computed tomography
- DWI-MR:
-
Magnetic resonance diffusion
- EPI:
-
Ultra-fast echoplanar imaging
- FDG:
-
F18-fluorodeoxyglucose
- HE:
-
Hematoxylin-Eosin
- HSA:
-
Areas of higher suspicion
- LSA:
-
Areas of lower suspicion
- MRI:
-
Magnetic resonance
- ND:
-
Non-diagnostic
- PET:
-
Positron emission tomography
- PET-CT:
-
Positron emission tomography-computed tomography
- SUV:
-
Standard uptake value
References
Geraghty PR, Kee ST, McFarlane G, Razavi MK, Sze DY, Dake MD (2003) CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology. 229(2):475–481. https://doi.org/10.1148/radiol.2291020499
Montaudon M, Latrabe V, Pariente A, Corneloup O, Begueret H, Laurent F (2004) Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol 14(7):1234–1240. https://doi.org/10.1007/s00330-004-2250-3
Yeow KM, Tsay PK, Cheung YC, Lui KW, Pan KT, Shau-Bin CA (2003) Factors affecting diagnostic accuracy of CT-guided coaxial cutting needle lung biopsy: Retrospective analysis of 631 procedures. J Vasc Interv Radiol 14(5):581–588. https://doi.org/10.1097/01.RVI.0000071087.76348.C7
Hiraki T, Mimura H, Gobara H et al (2009) CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles: diagnostic yield and risk factors for diagnostic failure. Chest. 136(6):1612–1617. https://doi.org/10.1378/chest.09-0370
Guimaraes MD, Marchiori E, Odisio BC et al (2014) Functional imaging with diffusion-weighted MRI for lung biopsy planning: initial experience. World J Surg Oncol 12(1). https://doi.org/10.1186/1477-7819-12-203
Barreto MM, Rafful PP, Rodrigues RS et al (2013) Correlation between computed tomographic and magnetic resonance imaging findings of parenchymal lung diseases. Eur J Radiol 82(9):e492–e501. https://doi.org/10.1016/j.ejrad.2013.04.037
Matoba M, Tonami H, Kondou T et al (2007) Lung carcinoma: diffusion- weighted MR imaging — preliminary evaluation with apparent diffusion. Radiology 243(2)
Uto T, Takehara Y, Nakamura Y et al (2009) Higher sensitivity and specificity for diffusion-weighted imaging of malignant lung lesions without apparent diffusion coefficient quantification. Radiology. 252(1):247–254. https://doi.org/10.1148/radiol.2521081195
Baysal T, Mutlu DY, Yologlu S (2009) Diffusion-weighted magnetic resonance imaging in differentiation of postobstructive consolidation from central lung carcinoma. Magn Reson Imaging 27(10):1447–1454. https://doi.org/10.1016/j.mri.2009.05.024
Weber G (1977) Enzymology of cancer cells (first of two parts). N Engl J Med 296(9):486–492. https://doi.org/10.1056/NEJM197703032960905
Zijlstra JM, Lindauer-van der Werf G, Hoekstra OS, Hooft L, Riphagen II, Huijgens PC (2006) 18F-fluoro-deoxyglucose positron emission tomography for post-treatment evaluation of malignant lymphoma: a systematic review. Haematologica 91(4):522–529
Guralnik L, Rozenberg R, Frenkel A et al (2015) Metabolic PET/CT-gided lung lesion biopsies:impact on diagnostic accuracy and rate of sampling error. J Nucl Med 56(4):518–522
Purandare NC, Kulkarni AV, Kulkarni SS et al (2013) 18F-FDG PET/CT-directed biopsy: does it offer incremental benefit? Nucl Med Commun 34(3):203–210
Jackson EL, Willis N, Mercer K et al (2001) Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 617:3243–3248. https://doi.org/10.1101/gad.943001
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to Gefitinib. N Engl J Med 350(21):2129–2139
Kris MG, Johnson BE, Berry LD et al (2014) Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311(19):1998–2006. https://doi.org/10.1001/jama.2014.3741
Kleppe M, Levine RL (2014) Tumor heterogeneity confounds and illuminates: assessing the implications. Nat Med 20(4):342–344. https://doi.org/10.1038/nm.3522
Marusyk A, Almendro V, Polyak K (2012) Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 12(5):323–334. https://doi.org/10.1038/nrc3261
Cooper RA, Carrington BM, Loncaster JA et al (2000) Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol 57(1):53–59 http://www.ncbi.nlm.nih.gov/pubmed/11033189
Foo SS, Abbott DF, Lawrentschuk N, Scott AM (2004) Functional imaging of intratumoral hypoxia. Mol Imaging Biol 6(5):291–305. https://doi.org/10.1016/j.mibio.2004.06.007
Weiss CR, Nour SG, Lewin JS (2008) MR-guided biopsy: a review of current techniques and applications. J Magn Reson Imaging 27(2):311–325. https://doi.org/10.1002/jmri.21270
Gould MK, Fletcher J, Iannettoni MD et al (2007) Evaluation of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132(3 SUPPL). https://doi.org/10.1378/chest.07-1353
Dhital K, Saunders CA, Seed PT, O’Doherty MJ, Dussek J (2000) [(18)F]Fluorodeoxyglucose positron emission tomography and its prognostic value in lung cancer. Eur J Cardiothorac Surg 18(4):425–428
Calais J, Dubray B, Nkhali L et al (2015) High FDG uptake areas on pre-radiotherapy PET/CT identify preferential sites of local relapse after chemoradiotherapy for locally advanced oesophageal cancer. Eur J Nucl Med Mol Imaging 42(6):858–867. https://doi.org/10.1007/s00259-015-3004-y
Paesmans M, Berghmans T, Dusart M et al (2010) Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol 5(5):612–619
Feng M, Yang X, Ma Q, He Y (2017) Retrospective analysis for the false positive diagnosis of PET-CT scan in lung cancer patients. Medicine (Baltimore) 96(42):e7415. https://doi.org/10.1097/MD.0000000000007415
Acknowledgments
C.E.Z. takes full responsibility for the content of the manuscript, including the data analysis. M.D.G. and R.C. contributed to conception and design. C.J.T., P.N.B., A.G.V.B., E.N.P.L., J.L.G., and C.A.L.P. contributed to patient selection, execution of the imaging, procedure, and sample analysis. A.C.B.S.C. and J.P.K.M.J. contributed to manuscript preparation.
Funding
This study has received funding by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (no. 2013/15143-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Charles Edouard Zurstrassen.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zurstrassen, C.E., Tyng, C.J., Guimarães, M.D. et al. Functional and metabolic imaging in transthoracic biopsies guided by computed tomography. Eur Radiol 30, 2041–2048 (2020). https://doi.org/10.1007/s00330-019-06591-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06591-0