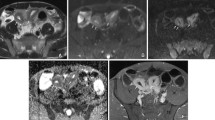
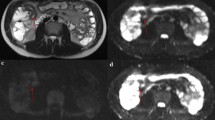
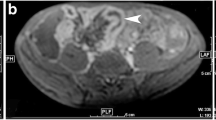
Abstract
Objectives
Identifying inflammation- or fibrosis-predominant strictures in Crohn’s disease (CD) is crucial for treatment strategies. We evaluated the additive value of magnetisation transfer (MT) to conventional MRI for differentiating CD strictures using surgical histopathology as a reference standard.
Methods
Twenty-eight consecutive CD patients who underwent MRI preoperatively were recruited. MRI parameters included T2-weighted imaging (T2WI) hyperintensity, bowel wall thickness, enhancement pattern changes over time, enhancement pattern and gain ratio in dynamic contrast-enhanced phases, and MT ratio. Correlation analysis was performed using Spearman’s rank test. Receiver operating characteristic curve analysis and Cohen’s κ were used. A model with combined MRI variables characterising intestinal strictures was proposed and validated in 14 additional CD patients.
Results
Significant correlations with histological inflammation scores were shown for wall thickness (r = 0.361, p = 0.001) and T2WI hyperintensity (r = 0.396, p < 0.001), whereas histological fibrosis scores were significantly correlated with MT ratio (r = 0.681, p < 0.001) and wall thickness (r = 0.461, p < 0.001). T2WI hyperintensity could differentiate mild from moderate-to-severe inflammation with a sensitivity of 0.871 and a specificity of 0.800. MT ratio could discriminate mild from moderate-to-severe fibrosis with a sensitivity and a specificity of 0.913 and 0.923, respectively. Combining MT ratio and T2WI hyperintensity, the MRI classification moderately agreed with the pathological stricture classification (p < 0.01, κ = 0.549). In the validation set, the diagnostic accuracy of T2WI hyperintensity and MT ratio were 86% and 89%, with good agreement between MRI and histopathological classification (p < 0.01, κ = 0.665).
Conclusions
MT ratio combined with conventional MRI improves the differentiation of fibrotic from inflammatory components of small-bowel strictures in CD patients.
Key Points
• MT ratio from magnetisation transfer imaging combined with T2WI from conventional MRI can simultaneously characterise bowel fibrosis and inflammation in adult Crohn’s disease.



Similar content being viewed by others
Abbreviations
- CD:
-
Crohn’s disease
- CDAI:
-
Crohn’s disease activity index
- CI:
-
Confidence interval
- H&E:
-
Haematoxylin and eosin
- ICC:
-
Intraclass correlation coefficient
- MT:
-
Magnetisation transfer
- MTI:
-
Magnetisation transfer imaging
- OR:
-
Odds ratio
- SI:
-
Signal intensity
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
References
Cosnes J, Cattan S, Blain A et al (2002) Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 8:244–250
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A (2011) Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140:1785–1794
Tielbeek JAW, Ziech MLW, Li Z et al (2014) Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol 24:619–629
Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS (2007) Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol 102:2541–2550
Adler J, Punglia DR, Dillman JR et al (2012) Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohnʼs disease. Inflamm Bowel Dis 18:849–856
Zappa M, Stefanescu C, Cazals-Hatem D et al (2011) Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis 17:984–993
Panes J, Bouhnik Y, Reinisch W et al (2013) Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis 7:556–585
Horsthuis K, Bipat S, Bennink RJ, Stoker J (2008) Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 247:64–79
Baumgart DC, Sandborn WJ (2012) Crohn’s disease. Lancet 380:1590–1605
Punwani S, Rodriguez-Justo M, Bainbridge A et al (2009) Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology 252:712–720
Rimola J, Planell N, Rodríguez S et al (2015) Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol 110:432–440
Wagner M, Ko HM, Chatterji M et al (2018) Magnetic resonance imaging predicts histopathologic composition of ileal Crohn’s disease. J Crohns Colitis 12:718–729
Fallis SA, Murphy P, Sinha R et al (2013) Magnetic resonance enterography in Crohn’s disease: a comparison with the findings at surgery. Colorectal Dis 15:1273–1280
Adler J, Swanson SD, Schmiedlin-Ren P et al (2011) Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn disease. Radiology 259:127–135
Adler J, Rahal K, Swanson SD et al (2013) Anti-tumor necrosis factor α prevents bowel fibrosis assessed by messenger RNA, histology, and magnetization transfer MRI in rats with Crohnʼs disease. Inflamm Bowel Dis 19:683–690
Dillman JR, Swanson SD, Johnson LA et al (2015) Comparison of noncontrast MRI magnetization transfer and T2-weighted signal intensity ratios for detection of bowel wall fibrosis in a Crohn’s disease animal model. J Magn Reson Imaging 42:801–810
Pinson C, Dolores M, Cruypeninck Y et al (2017) Magnetization transfer ratio for the assessment of perianal fistula activity in Crohn’s disease. Eur Radiol 27:80–87
Wolff SD, Balaban RS (1994) Magnetization transfer imaging: practical aspects and clinical applications. Radiology 192:593–599
Li XH, Mao R, Huang SY et al (2018) Characterization of degree of intestinal fibrosis in patients with Crohn disease by using magnetization transfer MR imaging. Radiology 287:494–503
Sandborn WJ, Feagan BG, Hanauer SB et al (2002) A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 122:512–530
Li XH, Sun CH, Mao R et al (2015) Assessment of activity of Crohn disease by diffusion-weighted magnetic resonance imaging. Medicine (Baltimore) 94:e1819
Pazahr S, Blume I, Frei P et al (2013) Magnetization transfer for the assessment of bowel fibrosis in patients with Crohn’s disease: initial experience. MAGMA 26:291–301
Catalano OA, Gee MS, Nicolai E et al (2016) Evaluation of quantitative PET/MR enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in Crohn disease. Radiology 278:792–800
Malagò R, Manfredi R, Benini L, D’Alpaos G, Mucelli RP (2008) Assessment of Crohn’s disease activity in the small bowel with MR-enteroclysis: clinico-radiological correlations. Abdom Imaging 33:669–675
Girometti R, Zuiani C, Toso F et al (2008) MRI scoring system including dynamic motility evaluation in assessing the activity of Crohn’s disease of the terminal ileum. Acad Radiol 15:153–164
Florie J, Wasser MN, Arts-Cieslik K, Akkerman EM, Siersema PD, Stoker J (2006) Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn’s disease. AJR Am J Roentgenol 186:1384–1392
Miao YM, Koh DM, Amin Z et al (2002) Ultrasound and magnetic resonance imaging assessment of active bowel segments in Crohn’s disease. Clin Radiol 57:913–918
Pupillo VA, Di Cesare E, Frieri G, Limbucci N, Tanga M, Masciocchi C (2007) Assessment of inflammatory activity in Crohn’s disease by means of dynamic contrast-enhanced MRI. Radiol Med 112:798–809
Ziech ML, Bipat S, Roelofs JJ et al (2011) Retrospective comparison of magnetic resonance imaging features and histopathology in Crohn’s disease patients. Eur J Radiol 80:e299–e305
Bruining DH, Zimmermann EM, Loftus EV et al (2018) Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Gastroenterology 154:1172–1194
Funding
This study has received funding by the National Natural Science Foundation of China (81600508, 81500501, 81770654, 81771908, 81571750, 81870451).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantors of this publication are Ren Mao and Shi-Ting Feng.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Supplementary Table 1 MRI sequences and parameters. Supplementary Table 2 Histologic score for inflammatory and fibrotic Crohn’s disease. Supplementary Table 3 Correlation between MRI parameters and histological inflammation score. Supplementary Table 4 Correlation between MRI parameters and histological fibrosis score. Supplementary Fig. 1 Mural SI was defined as normal (equal to the SI of normal bowel walls) or hyperintense (higher than the SI of normal bowel walls) on T2WI. Coronal T2WI demonstrates high signal intensity in the thickened intestinal wall (arrow), whereas the normal bowel wall showed low-signal intensity (arrowhead). SI signal intensity, T2WI T2-weighted imaging. Supplementary Fig. 2 The enhancement pattern was classified as mucosal only (with enhancement of only the innermost wall layer), homogeneous (with the entire bowel wall showing equal enhancement), or layered (with both mucosal and serosal bowel wall layers showing enhancement and with a central band of relatively reduced enhancement). (a) Precontrast T1-weighted image showing bowel wall thickening and luminal narrowing (arrow); (b-d) Coronal postcontrast magnetic resonance images of thickened bowel walls demonstrating (b) mucosal only, (c) layered, and (d) homogeneous patterns of enhancement. Arrow = bowel serosa, arrowhead = mucosa. Supplementary Fig. 3 The enhancement progressions from 70 s to 7 min in deep layers were recorded as stability over time (e.g., homogeneous, mucosal, or layered) and progression of enhancement over time (from mucosal or layered to homogeneous). The deeper layers of the bowel wall show lower enhancement than the superficial layers at 70 s after contrast injection (a, arrow) but homogenous enhancement at 7 min (b, arrow). Supplementary Fig. 4 Axial MT imaging without (a) and with (b) MT pulse. The off-resonance prepulse had a Gaussian shape with a frequency offset of 1.2 kHz, a duration of 9984 μs, an effective flip angle of 500°, and a bandwidth of 192 Hz. MT magnetization transfer. Supplementary Fig. 5 Pairwise comparison shows a significantly higher MT ratio in moderate inflammation than in mild inflammation (p = 0.001). There are no significant differences between moderate and severe inflammation (p = 1.000) or between mild and severe inflammation (p = 0.062). Supplementary Fig. 6 Pairwise comparison shows that wall thickness is significantly lower in mild inflammation than in severe (p < 0.001) and moderate (p < 0.001) inflammation. There is no significant difference between moderate and severe inflammation (p = 1.000). Supplementary Fig. 7 Pairwise comparison shows a significantly higher MT ratio in severe fibrosis than in moderate (p = 0.005) and mild (p < 0.001) fibrosis. The MT ratio of moderate fibrosis is also significantly higher than that of mild fibrosis (p < 0.001). Supplementary Fig. 8 Pairwise comparison shows that wall thickness is significantly lower in mild fibrosis than in severe (p < 0.001) and moderate (p < 0.001) fibrosis. There is no significant difference between moderate and severe fibrosis (p = 0.167) (DOCX 41325 kb) (DOCX 41325 kb)
Rights and permissions
About this article
Cite this article
Fang, Zn., Li, Xh., Lin, Jj. et al. Magnetisation transfer imaging adds information to conventional MRIs to differentiate inflammatory from fibrotic components of small intestinal strictures in Crohn’s disease. Eur Radiol 30, 1938–1947 (2020). https://doi.org/10.1007/s00330-019-06594-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06594-x