Abstract
Background
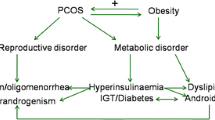
Polycystic ovary syndrome (PCOS), a common hormonal disorder in women, affects 4–18% of women of reproductive age worldwide. A higher prevalence of irritable bowel syndrome was found in women with PCOS. However, the effects and mechanism of PCOS on stomach and colon contractility remain unclear.
Aims
This study aims to evaluate the correlation between PCOS and gastrointestinal disorder.
Methods
Four-week-old female rats were subcutaneously implanted with pellets containing 7.5 mg of dihydrotestosterone for 13 weeks to create PCOS rat models. After vaginal smears, the estrus cycle stage was evaluated. Oral glucose tolerance test was performed after 90 days of treatment. All animals were killed at 17 weeks. The rats were fasted overnight and then anesthetized before decapitation, and the stomach fundus and colon were surgically removed and cultured in oxygenated Krebs solution. Acetylcholine and carbachol were used to evaluate the cholinergic system on contractility.
Results
The basal and stomach fundus responded with a reduced frequency and contractility in response to acetylcholine in the PCOS group. Moreover, no difference was found in the spontaneous stomach contractility induced by carbachol in both groups. Lower maximal colon muscle contractility was also found in response to acetylcholine stimulation in PCOS rats. Furthermore, lower maximal muscle contractility was found in response to extracellular calcium levels. MLC20 phosphorylation was also reduced in the gastrointestinal tissue in PCOS rats.
Conclusions
PCOS induces gastroparesis and reduces gastrointestinal muscle contractility. This effect is, at least partly, through reducing the responsiveness of acetylcholine and MLC20 phosphorylation.






Similar content being viewed by others
Change history
20 April 2020
The original version of the article unfortunately contained an error in the legend of Figure��5B. Corrected version of Figure��5 is given below.
References
Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility—insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2016;9:633–645.
Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2:650–656.
Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastric function. Neurogastroenterol Motil. 22:1154-1163.
Saji M, Miura M. Nicotinic and muscarinic property of ACh receptors on cultured brain stem neurons. Jpn J Physiol. 1982;32:1015–1021.
Scarr E. Muscarinic receptors: their roles in disorders of the central nervous system and potential as therapeutic targets. CNS Neurosci Ther. 2012;18:369–379.
Luo W, Latchney LR, Culp DJ. G protein coupling to M1 and M3 muscarinic receptors in sublingual glands. Am J Physiol Cell Physiol. 2001;280:C884–C896.
Lin S, Kajimura M, Takeuchi K, Kodaira M, Hanai H, Kaneko E. Expression of muscarinic receptor subtypes in rat gastric smooth muscle: effect of M3 selective antagonist on gastric motility and emptying. Dig Dis Sci. 1997;42:907–914. https://doi.org/10.1023/a:1018808329603
Ohki Y, Tomomasa T, Tabata M, et al. Delayed gastric emptying in a neonate, associated with a partial defect in the gastric smooth muscle.J Pediatr Surg. 1995;30:1511–1512.
Motta AB. The role of obesity in the development of polycystic ovary syndrome. Curr Pharm Des. 2012;18:2482–2491.
DeVane GW, Czekala NM, Judd HL, Yen SS. Circulating gonadotropins, estrogens, and androgens in polycystic ovarian disease. Am J Obstet Gynecol. 1975;121:496–500.
Fleming R, McQueen D, Yates RW, Coutts JR. Spontaneous follicular and luteal function in infertile women with oligomenorrhoea: role of luteinizing hormone. Clin Endocrinol (Oxf). 1995;43:735–739.
Morales AJ, Laughlin GA, Butzow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996;81:2854–2864.
Huang G, Coviello A. Clinical update on screening, diagnosis and management of metabolic disorders and cardiovascular risk factors associated with polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2012;19:512–519.
Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812–841.
Chang FY, Doong ML, Chen TS, Lee SD, Wang PS. Altered intestinal transit is independent of gastroparesis in the early diabetic rats. Chin J Physiol. 1997;40:31–35.
Horowitz M, Edelbroek M, Fraser R, Maddox A, Wishart J. Disordered gastric motor function in diabetes mellitus. Recent insights into prevalence, pathophysiology, clinical relevance, and treatment. Scand J Gastroenterol. 1991;26:673-684.
Dubois A. Gastric dysrhythmias: pathophysiologic and etiologic factors. Mayo Clin Proc. 1989;64:246–250.
Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment.Dig Dis Sci. 1999;44:1061–1075. https://doi.org/10.1023/a:1026647417465
Rothstein RD. Gastrointestinal motility disorders in diabetes mellitus. Am J Gastroenterol. 1990;85:782–785.
Takahashi T, Matsuda K, Kono T, Pappas TN. Inhibitory effects of hyperglycemia on neural activity of the vagus in rats. Intensive Care Med. 2003;29:309–311.
Huang W, Hung C, Chen M, et al. Involvement of cyclooxygenase 2 and prostagladin E(2) in the effects of insulin on gastric emptying in male rats.J Physiol Pharmacol. 2009;60:109–118.
Mahajan DK. Polycystic ovarian disease: animal models. Endocrinol Metab Clin North Am. 1988;17:705–732.
Ngadjui E, Watcho P, Nguelefack TB, Kamanyi A, Carro-Juarez M. Effects of Ficus asperifolia on normal rat estrus cyclicity. Asian Pac J Trop Biomed. 2013;3:53–57.
Lo MJ, Kau MM, Wang PS. Effect of aging on corticosterone secretion in diestrous rats. J Cell Biochem. 2006;97:351–358.
Mandl AM, Zuckerman S. The time of vaginal opening in rats after ovarian autotransplantation. J Endocrinol. 1951;7:335–338.
Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1973;8:491–494.
Wu JJ, Wang KL, Wang SW, et al. Differential effects of nonylphenol on testosterone secretion in rat Leydig cells. Toxicology. 2010;268:1–7.
Wang KL, Hsia SM, Mao IF, Chen ML, Wang SW, Wang PS. Effects of polybrominated diphenyl ethers on steroidogenesis in rat Leydig cells. Hum Reprod. 2011;26:2209–2217.
Hsia SM, Wang KL, Wang PS. Effects of resveratrol, a grape polyphenol, on uterine contraction and Ca(2) + mobilization in rats in vivo and in vitro. Endocrinology. 2011;152:2090–2099.
Touw K, Chakraborty S, Zhang W, et al. Altered calcium signaling in colonic smooth muscle of type 1 diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G66–G76.
Evans ED, Mangel AW. Depolarization-stimulated contractility of gastrointestinal smooth muscle in calcium-free solution: a review. ISRN Gastroenterol. 2011;2011:692528.
Wang KL, Hsia SM, Chan CJ et al.. Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells.Expert Opin Ther Targets. 2013:337-349.
Mathur R, Ko A, Hwang LJ, Low K, Azziz R, Pimentel M. Polycystic ovary syndrome is associated with an increased prevalence of irritable bowel syndrome. Dig Dis Sci. 2010;55:1085–1089. https://doi.org/10.1007/s10620-009-0890-5
Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961–4970.
Hasler WL. Gastroparesis–current concepts and considerations.Medscape J Med. 2008;10:16.
Sadiya A. Nutritional therapy for the management of diabetic gastroparesis: clinical review. Diabetes Metab Syndr Obes. 2012;5:329–335.
Vanormelingen C, Tack J, Andrews CN. Diabetic gastroparesis.Br Med Bull. 2013;105:213–230.
Furlan MM, Molinari SL, Miranda Neto MH. Morphoquantitative effects of acute diabetes on the myenteric neurons of the proximal colon of adult rats.Arq Neuropsiquiatr. 2002;60:576–581.
Matos JF, Americo MF, Sinzato YK, et al. Role of sex hormones in gastrointestinal motility in pregnant and non-pregnant rats. World J Gastroenterol. 2016;22:5761–5768.
Osuka S, Iwase A, Nakahara T, et al. Kisspeptin in the Hypothalamus of 2 Rat Models of Polycystic Ovary Syndrome. Endocrinology. 2017;158:367–377.
Manneras L, Cajander S, Holmang A, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791.
Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats.Am J Physiol. 1995;268:G171–G176.
Hellstrom PM. Glucagon-like peptide-1 gastrointestinal regulatory role in metabolism and motility. Vitamins and Hormones. 2010;84:319–329.
Ghayur MN, Khan AH, Gilani AH. Ginger facilitates cholinergic activity possibly due to blockade of muscarinic autoreceptors in rat stomach fundus. Pak J Pharm Sci. 2007;20:231–235.
Proctor GB. Muscarinic receptors and salivary secretion. J Appl Physiol. 2006;100:1103–1104.
Yamashita T, Kokubun S. Calcium channel modulation by M3 receptor in guinea-pig tracheal smooth muscle cells. Jpn J Pharmacol. 1992;58:404P.
Funding
This study was funded by a grant (107-2320-B-254 -001) from the National Science Council, Taipei, Taiwan, ROC.
Author information
Authors and Affiliations
Contributions
Study concepts, study design, data acquisition, data analysis and interpretation, statistical analysis, and manuscript preparation: K.-L. Wang. Animal preparation: P.-H. Lin. Manuscript editing and review: S.-M. Hsia and P. S. Wang.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, KL., Hsia, SM., Wang, P.S. et al. Disturbed Gastrointestinal Contractility in a Polycystic Ovary Syndrome Rat Model. Dig Dis Sci 65, 2834–2843 (2020). https://doi.org/10.1007/s10620-019-06001-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-06001-x