Abstract
Objectives
In this clinical study, we aim to evaluate the effectiveness of non-thermal atmospheric pressure plasma (NAPP), which is a novel procedure used in periodontal pocket decontamination adjunctive to non-surgical periodontal treatment (NSPT).
Methods
The study included 25 systemically healthy periodontitis patients. In the split-mouth design, NAPP application into the pockets, in addition to NSPT, was performed. Clinical periodontal data, gingival crevicular fluid, and subgingival plaque samples of patients were taken before and during the first and third months of treatment. Biochemical assays were conducted using enzyme-linked immunosorbent assay. Analysis of bacteria was performed with polymerase chain reaction method.
Results
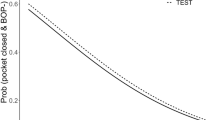
There was more clinical attachment level (CAL) gain in the 3rd month in the test group (deep pockets: 3.90 mm, pockets ≥ 5 mm: 2.72 mm) compared to the control group (deep pockets: 3.40 mm, pockets ≥ 5 mm: 2.58 mm) (p < 0.05), but no significant difference between groups in CAL. Clinical periodontal parameters improved in both study groups (p < 0.05). However, the gingival index (GI) and the bleeding on probing (BOP) rate decreased more in the test group (GI: 0.55, BOP: 9.48%, and GI: 0.38, BOP: 8.46% in the 1st and 3rd months, respectively) compared to the control group (GI: 0.68, BOP: 13.43%, and GI: 0.52, BOP: 14.58%) (p < 0.05). In addition, there was no significant difference in probing depth and biochemical markers between groups (p > 0.05). It was observed that NAPP reduced the number of bacteria more than the control group in the 1st and 3rd months.
Conclusions
It was seen that the single-time NAPP application concurrent with NSPT provided additional CAL gain, elimination of putative periodontopathogens and reduced their recolonization. Longitudinal studies with larger population and longer time are required.
Clinical relevance
NSPT is an effective method for the treatment of periodontitis but bacteria recolonization that causes recurrence of the periodontal disease occurs within a short period. NAPP can reduce the recurrence of periodontal disease by providing better bacterial elimination and should, therefore, be used in maintenance of periodontitis.




Similar content being viewed by others

References
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F (2018) Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol 89:S173–S182
Mombelli A (2018) Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000(76):85–96. https://doi.org/10.1111/prd.12147
Lang NP, Salvi GE, Sculean A (2019) Nonsurgical therapy for teeth and implants—when and why? Periodontology 79:15–21. https://doi.org/10.1111/prd.12240
Giuliana G, Ammatuna P, Pizza G, Capone F, D'angelo M (1997) Occurrence of invading bacteria in radicular dentin of periodontally diseased teeth: microbiological findings. J Clin Periodontol 24:478–485
Tribble GD, Lamont RJ (2010) Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol 2000(52):68–83
Jepsen K, Jepsen S (2016) Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontology 2000(71):82–112. https://doi.org/10.1111/prd.12121
Karlsson MR, Diogo Löfgren CI, Jansson HM (2008) The effect of laser therapy as an adjunct to non-surgical periodontal treatment in subjects with chronic periodontitis: a systematic review. J Periodontol 79:2021–2028
Sgolastra F, Petrucci A, Severino M, Graziani F, Gatto R, Monaco A (2013) Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol 40:514–526
John MT, Michalowicz BS, Kotsakis GA, Chu H (2017) Network meta-analysis of studies included in the clinical practice guideline on the nonsurgical treatment of chronic periodontitis. J Clin Periodontol
Heinlin J, Morfill G, Landthaler M, Stolz W, Isbary G, Zimmermann JL, Shimizu T, Karrer S (2010) Plasma medicine: possible applications in dermatology. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 8:968–976
Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A (2008) Applied plasma medicine. Plasma Process Polym 5:503–533
Weltmann KD, von Woedtke T (2016) Plasma medicine—current state of research and medical application. Plasma Physics and Controlled Fusion 59:014031. https://doi.org/10.1088/0741-3335/59/1/014031
Yildirim ED, Ayan H, Vasilets VN, Fridman A, Guceri S, Sun W (2008) Effect of dielectric barrier discharge plasma on the attachment and proliferation of osteoblasts cultured over poly (ε-caprolactone) scaffolds. Plasma Process Polym 5:58–66
Ritts AC, Li H, Yu Q, Xu C, Yao X, Hong L, Wang Y (2010) Dentin surface treatment using a non-thermal argon plasma brush for interfacial bonding improvement in composite restoration. Eur J Oral Sci 118:510–516
Dong X, Ritts AC, Staller C, Yu Q, Chen M, Wang Y (2013) Evaluation of plasma treatment effects on improving adhesive–dentin bonding by using the same tooth controls and varying cross-sectional surface areas. Eur J Oral Sci 121:355–362
Karaman O, Kelebek S, Demirci EA, İbiş F, Ulu M, Ercan UK (2018) Synergistic effect of cold plasma treatment and RGD peptide coating on cell proliferation over titanium surfaces. Tissue Eng Regen Med 15:13–24
Maisch T, Shimizu T, Li Y-F, Heinlin J, Karrer S, Morfill G, Zimmermann JL (2012) Decolonisation of MRSA, S. aureus and E. coli by cold-atmospheric plasma using a porcine skin model in vitro. PLoS One 7:e34610
Seo S-H, Han I, Lee HS, Choi JJ, Choi EH, Kim K-N, Park G, Kim K-M (2017) Antibacterial activity and effect on gingival cells of microwave-pulsed non-thermal atmospheric pressure plasma in artificial saliva. Sci Rep 7:8395
Ermolaeva SA, Varfolomeev AF, Chernukha MY, Yurov DS, Vasiliev MM, Kaminskaya AA, Moisenovich MM, Romanova JM, Murashev AN, Selezneva II (2011) Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J Med Microbiol 60:75–83
Isbary G, Morfill G, Schmidt H, Georgi M, Ramrath K, Heinlin J, Karrer S, Landthaler M, Shimizu T, Steffes B (2010) A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol 163:78–82
Bekeschus S, von Woedtke T, Kramer A, Weltmann K-D, Masur K (2013) Cold physical plasma treatment alters redox balance in human immune cells. Plasma Med 3
Lee OJ, Ju HW, Khang G, Sun PP, Rivera J, Cho JH, Park SJ, Eden JG, Park CH (2016) An experimental burn wound-healing study of non-thermal atmospheric pressure microplasma jet arrays. J Tissue Eng Regen Med 10:348–357
Kwon J-S, Kim YH, Choi EH, Kim C-K, Kim K-N, Kim K-M (2016) Non-thermal atmospheric pressure plasma increased mRNA expression of growth factors in human gingival fibroblasts. Clin Oral Investig 20:1801–1808
Kalghatgi S, Friedman G, Fridman A, Clyne AM (2010) Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann Biomed Eng 38:748–757
Arndt S, Landthaler M, Zimmermann JL, Unger P, Wacker E, Shimizu T, Li Y-F, Morfill GE, Bosserhoff A-K, Karrer S (2015) Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS One 10:e0120041
Nakajima Y, Mukai K, Rahayu HSE, Nur M, Ishijima T, Enomoto H, Uesugi Y, Sugama J, Nakatani T (2014) Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin Plasma Med 2:28–35
Haertel B, von Woedtke T, Weltmann K-D, Lindequist U (2014) Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol Ther (Seoul) 22:477
Heinlin J, Zimmermann JL, Zeman F, Bunk W, Isbary G, Landthaler M, Maisch T, Monetti R, Morfill G, Shimizu T (2013) Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair Regen 21:800–807
Brehmer F, Haenssle H, Daeschlein G, Ahmed R, Pfeiffer S, Görlitz A, Simon D, Schön M, Wandke D, Emmert S (2015) Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm® VU-2010): results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J Eur Acad Dermatol Venereol 29:148–155
Tomasi C, Schander K, Dahlen G, Wennstrom JL (2006) Short-term clinical and microbiologic effects of pocket debridement with an Er:YAG laser during periodontal maintenance. J Periodontol 77:111–118. https://doi.org/10.1902/jop.2006.77.1.111
Sanz-Sanchez I, Ortiz-Vigon A, Herrera D, Sanz M (2016) Microbiological effects and recolonization patterns after adjunctive subgingival debridement with Er:YAG laser. Clin Oral Investig 20:1253–1261. https://doi.org/10.1007/s00784-015-1617-y
Silness J, Löe H (1964) Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 22:121–135
Löe H, Silness J (1963) Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol Scand 21:533–551
Ainamo J, Bay I (1975) Problems and proposals for recording gingivitis and plaque. Int Dent J 25:229–235
Karaman O, Kumar A, Moeinzadeh S, He X, Cui T, Jabbari E (2016) Effect of surface modification of nanofibres with glutamic acid peptide on calcium phosphate nucleation and osteogenic differentiation of marrow stromal cells. J Tissue Eng Regen Med 10
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Shi Q, Song K, Zhou X, Xiong Z, Du T, Lu X, Cao Y (2015) Effects of non-equilibrium plasma in the treatment of ligature-induced peri-implantitis. J Clin Periodontol 42:478–487
Pérez-Chaparro PJ, Duarte PM, Shibli JA, Montenegro S, Lacerda Heluy S, Figueiredo LC, Faveri M, Feres M (2016) The current weight of evidence of the microbiologic profile associated with peri-implantitis: a systematic review. J Periodontol 87:1295–1304
Holt SC, Ebersole JL (2005) Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000(38):72–122
Umeda M, Takeuchi Y, Noguchi K, Huang Y, Koshy G and Ishikawa I (2004) Effects of nonsurgical periodontal therapy on the microbiota. Periodontol 2000 36:98–120
Cobb CM (1996) Non-surgical pocket therapy: mechanical. Ann Periodontol 1:443–490
Sbordone L, Ramaglia L, Gulletta E, Iacono V (1990) Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J Periodontol 61:579–584
Soeroso Y, Akase T, Sunarto H, Kemal Y, Salim R, Octavia M, Viandita A, Setiawan J, Bachtiar B (2017) The risk reduction of recurrent periodontal pathogens of local application minocycline hCl 2% gel, used as an adjunct to scaling and root planing for chronic periodontitis treatment. Ther Clin Risk Manag 13:307
Kovalova Z, Zahoran M, Zahoranová A, Machala Z (2014) Streptococci biofilm decontamination on teeth by low-temperature air plasma of dc corona discharges. J Phys D Appl Phys 47:224014
Shekhter AB, Serezhenkov VA, Rudenko TG, Pekshev AV, Vanin AF (2005) Beneficial effect of gaseous nitric oxide on the healing of skin wounds. Nitric Oxide 12:210–219
Zoellner H, Chapple CC, Hunter N (2002) Microvasculature in gingivitis and chronic periodontitis: disruption of vascular networks with protracted inflammation. Microsc Res Tech 56:15–31
Hou LT, Liu CM, Rossomando EF (1995) Crevicular interleukin-1β in moderate and severe periodontitis patients and the effect of phase I periodontal treatment. J Clin Periodontol 22:162–167
Prapulla DV, Sujatha PB, Pradeep A (2007) Gingival crevicular fluid VEGF levels in periodontal health and disease. J Periodontol 78:1783–1787
Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A (2000) Levels of interleukin-1β,-8, and-10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol 71:1535–1545
Choi J-H, Lee H-J, Hong J-W, Kim G-C (2015) The treatment with non-thermal plasma on human keratinocyte can block TNF-α and IFN-γ mediated pro-inflammatory gene expressions. Book title, IEEE
Smail-Faugeron V, Fron-Chabouis H, Courson F, Durieux P (2014) Comparison of intervention effects in split-mouth and parallel-arm randomized controlled trials: a meta-epidemiological study. BMC Med Res Methodol 14:64. https://doi.org/10.1186/1471-2288-14-64
Husejnagic S, Lettner S, Laky M, Georgopoulos A, Moritz A, Rausch-Fan X (2019) Photoactivated disinfection in periodontal treatment: a randomized controlled clinical split-mouth trial. J Periodontol
Berakdar M, Callaway A, Eddin MF, Roß A, Willershausen B (2012) Comparison between scaling-root-planing (SRP) and SRP/photodynamic therapy: six-month study. Head Face Med 8:12
Smiley CJ, Tracy SL, Abt E, Michalowicz BS, John MT, Gunsolley J, Cobb CM, Rossmann J, Harrel SK, Forrest JL (2015) Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc 146:508–524.e5
Megally A, Zekeridou A, Cancela J, Giannopoulou C, Mombelli A (2019) Short ultrasonic debridement with adjunctive low-concentrated hypochlorite/amino acid gel during periodontal maintenance: randomized clinical trial of 12 months. Clin Oral Investig:1–9
Funding
This study was funded by The Scientific and Technological Research Council of Turkey (TÜBİTAK) with the project number 315S274.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This prospective, split-mouth, double-masked, placebo controlled randomized clinical trial (RCT) was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2008, and the study protocol was approved by the Research Ethics Committee of İzmir Katip Çelebi University of Medical Sciences (Date:16.09.2015, version number:1). This study was registered at Turkish Medicines And Medical Devices Agency (number: 71146310 [2015-AC-CE-00129]).
Informed consent
Informed consent was obtained from all patients in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Küçük, D., Savran, L., Ercan, U.K. et al. Evaluation of efficacy of non-thermal atmospheric pressure plasma in treatment of periodontitis: a randomized controlled clinical trial. Clin Oral Invest 24, 3133–3145 (2020). https://doi.org/10.1007/s00784-019-03187-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03187-2