Abstract
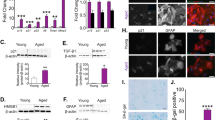
The aging brain is associated with significant pathophysiological changes reflected in changes in astrocyte function. In this study, we hypothesized that the response of astrocytes to mechanical and inflammatory stimulation would differ with long-term culture. We report that naïve short-term cultured (young) and long-term cultured astrocytes (aged) exhibit similar recovery to a scratch wound assay. However, in response to IL-1β young astrocytes have an arrested recovery which is not observed in IL-1β treated aged astrocytes. We had recently reported that astrocytes release extracellular vesicles (EVs) in response to IL-1β treatment. Given the disparate phenotypes between young and aged astrocytes, we next examined whether the EVs released from astrocytes reflected the differences in cellular responses to scratch and IL-1B treatment. Young cultures challenged with EVs collected from IL-1β treated cells exhibited a robust inhibition of wound recovery when compared to astrocytes treated with EVs collected from IL-1β treated aged astrocyte cultures. Heterochronic experiments also determined that the effect of IL-1β on astrocyte scratch wound recovery could be recapitulated by EVs alone. Taken together, these findings provide new information on how senescence alters the functional response and how EVs from astrocytes may elicit changes in glial responses which may have relevance to understanding neurological diseases.







Similar content being viewed by others
References
Volterra A, Meldolesi J (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nature Rev Neurosci 6:626–640
Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60:430–440
Cohen J, Torres C (2019) Astrocyte senescence: evidence and significance. Aging Cell 18:e12937
Bitto A et al (2010) Stress-induced senescence in human and rodent astrocytes. Exp Cell Res 316:2961–2968
Campuzano O, Castillo-Ruiz MM, Acarin L, Castellano B, Gonzalez B (2009) Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J Neurosci Res 87:2484–2497
Pertusa M, Garcia-Matas S, Rodriguez-Farre E, Sanfeliu C, Cristofol R (2007) Astrocytes aged in vitro show a decreased neuroprotective capacity. J Neurochem 101:794–805
Salminen A et al (2011) Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci 34:3–11
Tkach M, Thery C (2016) Communication by extracellular vesicles: where we are and where we need to go. Cell 164:1226–1232
Tkach M, Kowal J, Thery C (2018) Why the need and how to approach the functional diversity of extracellular vesicles. Philos Trans R Soc Lond B Biol Sci 373:20160479
Rashed H et al (2017) Exosomes: from garbage bins to promising therapeutic targets. Int J Mol Sci 18:538
Rojas et al (2018) DPTIP, a newly identified potent brain penetrant neutral sphingomyelinase 2 inhibitor, regulates astrocyte-peripheral immune communication following brain inflammation. Sci Rep 8:17715
Dickens AM et al (2017) Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signaling 10:7696
Chen Y, Tang Y, Fan GC, Duan DD (2018) Extracellular vesicles as novel biomarkers and pharmaceutic targets of diseases. Acta Pharmacol Sin 39:499–500
Cheng L, Sharples RA, Scicluna BJ, Hill AF (2014) Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles 3:23743
Chen G et al (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560:382–386
Willis CM et al (2017) A refined bead-free method to identify astrocytic exosomes in primary glial cultures and blood plasma. Front Neurosci 11:335
Crocker SJ, Milner R, Pham-Mitchell N, Campbell IL (2006) Cell and agonist-specific regulation of genes for matrix metalloproteinases and their tissue inhibitors by primary glial cells. J Neurochem 98:812–823
Johnson KM, Crocker SJ (2015) TIMP-1 couples RhoK activation to IL-1beta-induced astrocyte responses. Neurosci Lett 609:165–170
Johnson KM, Milner R, Crocker SJ (2015) Extracellular matrix composition determines astrocyte responses to mechanical and inflammatory stimuli. Neurosci Lett 600:104–109
Willis CM et al (2019) Extracellular vesicle fibrinogen induces encephalitogenic CD8+ T cells in a mouse model of multiple sclerosis. Proc Natl Acad Sci USA 116:10488–10493
Kawano H et al (2012) Long-term culture of astrocytes attenuates the readily releasable pool of synaptic vesicles. PLoS ONE 7:e48034
Hayflick L (1965) The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37:614–636
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Baker DJ et al (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530:184–189
Bussian TJ et al (2018) Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562:578–582
Jeon OH et al (2017) Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 23:775–781
Alcorta DA et al (1996) Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA 93:13742–13747
Stein GH, Drullinger LF, Soulard A, Dulic V (1999) Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol 19:2109–2117
Qian Y, Chen X (2013) Senescence regulation by the p53 protein family. Methods Mol Biol (Clifton, N.J.) 965:37–61
Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Ann Rev Pathol 5:99–118
Wiley CD et al (2016) Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabol 23:303–314
Malaquin N, Martinez A, Rodier F (2016) Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Exp Gerontol 82:39–49
Arriola Apelo SI, Lamming DW (2016) Rapamycin: an inhibitor of aging emerges from the soil of Easter Island. J Gerontol A Biol Sci Med Sci 71:841–849
Powell JD, Pollizzi KN, Heikamp EB, Horton MR (2012) Regulation of immune responses by mTOR. Annu Rev Immunol 30:39–68
Wang R, Sunchu B, Perez VI (2017) Rapamycin and the inhibition of the secretory phenotype. Exp Gerontol 94:89–92
Wang R et al (2017) Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 16:564–574
van der Loo B, Fenton MJ, Erusalimsky JD (1998) Cytochemical detection of a senescence-associated beta-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res 241:309–315
Itahana K, Campisi J, Dimri GP (2007) Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol (Clifton, N.J.) 371:21–31
Demaria M et al (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31:722–733
Bhat R et al (2012) Astrocyte senescence as a component of Alzheimer's disease. PLoS ONE 7:e45069
Zhang P et al (2019) Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat Neurosci. https://doi.org/10.1038/s41593-019-0372-9
Nicaise AM et al (2019) Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1818348116
Clarke LE et al (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA 115:e1896–e1905
Verkhratsky A, Nedergaard M (2018) Physiology of astroglia. Physiol Rev 98:239–389
Foerster S, Hill MFE, Franklin RJM (2019) Diversity in the oligodendrocyte lineage: plasticity or heterogeneity? Glia 67:1797–1805
Laberge RM, Awad P, Campisi J, Desprez PY (2012) Epithelial–mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron 5:39–44
Acosta JC et al (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133:1006–1018
Coppe JP et al (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6:2853–2868
Borghesan M et al (2019) Small extracellular vesicles are key regulators of non-cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep 27:3956–3971.e3956
Silverman JM et al (2019) CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem 294:3744–3759
Pei X, Li Y, Zhu L, Zhou Z (2019) Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp Cell Res 382:111474
Chistiakov DA, Chistiakov AA (2017) Alpha-synuclein-carrying extracellular vesicles in Parkinson's disease: deadly transmitters. Acta Neurol Belg 117:43–51
Goetzl EJ et al (2015) Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85:40–47
Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D (2018) High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol 83:544–552
Gao J et al (2019) Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol (Oxf). https://doi.org/10.1111/apha.13339
Watson LS, Hamlett ED, Stone TD, Sims-Robinson C (2019) Neuronally derived extracellular vesicles: an emerging tool for understanding Alzheimer's disease. Mol Neurodegener 14:22
Madill M et al (2017) Amyotrophic lateral sclerosis patient iPSC-derived astrocytes impair autophagy via non-cell autonomous mechanisms. Mol Brain 10:22
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special issue: In Honor of Professor Vittorio Gallo.
Rights and permissions
About this article
Cite this article
Willis, C.M., Sutter, P., Rouillard, M. et al. The Effects of IL-1β on Astrocytes are Conveyed by Extracellular Vesicles and Influenced by Age. Neurochem Res 45, 694–707 (2020). https://doi.org/10.1007/s11064-019-02937-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02937-8