Abstract
Objective
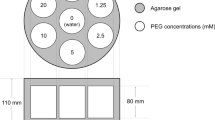
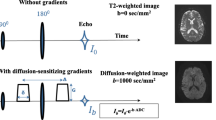
A phantom for diffusion-weighted imaging is required to standardize quantitative evaluation. The objectives were to develop a phantom simulating various cell densities and to evaluate repeatability.
Materials and methods
The acrylic fine particles with three different diameters were used to simulate human cells. Four-degree cell density components were developed by adjusting the volume of 10-μm particles (5, 20, 35, and 50% volume, respectively). Two-degree components to simulate cell edema were also developed by adjusting the diameter without changing number (17% and 40% volume, respectively). Spearman’s rank correlation coefficient was used to find a significant correlation between apparent diffusion coefficient (ADC) and particle density. Coefficient of variation (CV) for ADC was calculated for each component for 6 months. A p value < 0.05 represented a statistically significance.
Results
Each component (particle ratio of 5, 17, 20, 35, 40, and 50% volume, respectively) presented ADC values of 1.42, 1.30, 1.30, 1.12, 1.09, and 0.89 (× 10−3 mm2/s), respectively. A negative correlation (r = − 0.986, p < 0.05) was observed between ADC values and particle ratio. CV for ADC was less than 5%.
Discussion
A phantom simulating the diffusion restriction correlating with cell density and size could be developed.






Similar content being viewed by others
Change history
14 February 2020
The original version of this article unfortunately contained a mistake. Second column of “Cell edema” should read as.
Abbreviations
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- CV:
-
Coefficient of variation
- SD:
-
Standard deviation
References
Schaefer PW, Grant PE, Gonzalez RG (2000) Diffusion-weighted MR imaging of the brain. Radiology 217:331–345
Tien RD, Felsberg GJ, Friedman H, Brown M, MacFall J (1994) MR imaging of high-grade cerebral gliomas: value of diffusion weighted echo planar pulse sequences. AJR 162:671–677
Okamoto K, Ito J, Ishikawa K, Sakai K, Tokiguchi S (2000) Diffusion-weighted echo-planar MR imaging in differentiation diagnosis of brain tumors and tumor-like conditions. Eur Radiol 10:1342–1350
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR 188:1622–1635
Kolff-Gart AS, Pouwels PJW, Noji DP et al (2015) Diffusion-weighted imaging of the head and neck in healthy subjects: reproducibility of ADC values in different MRI systems and repeat sessions. AJNR 36:384–390
Braithwaite AC, Dale BM, Boll DT, Merkle EM (2009) Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology 250:459–465
Kivrak AS, Paksoy Y, Erol C, Koplay M, Ozbek S, Kara F (2013) Comparison of apparent diffusion coefficient values among different MRI platforms: a multicenter phantom study. Diagn interv Radiol 19:433–437
Sasaki M, Yamada K, Watanabe Y et al (2008) Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology 249:624–630
Malyarenko DI, Newitt D, Wilmes LJ et al (2016) Demonstration of nonlinearity bias in the measurement of the apparent diffusion coefficient in multicenter trials. Magn Reson Med 75:1312–1323
Lavdas I, Behan KC, Papadaki A, McRobbie DW, Aboagye EO (2013) A phantom for diffusion-weighted MRI (DW-MRI). J Magn Reson Imaging 38:173–179
Matsuya R, Kuroda M, Matsumoto Y et al (2009) A new phantom using polyethylene glycol as an apparent diffusion coefficient standard for MR imaging. Int J Oncol 35:893–900
Gatidis S, Schmidt H, Martirosian P, Schwenzer NF (2014) Development of an MRI phantom for diffusion-weighted imaging with independent adjustment of apparent diffusion coefficient values and T2 relaxation times. Magn Reson Med 72:459–463
Chenevert TL, Galban CJ, Ivancevic MK et al (2011) Diffusion coefficient measurement using a temperature-controlled fluid for quality control in multicenter studies. J Magn Reson Imaging 34:983–987
Malyarenko D, Galban CJ, Londy FJ et al (2013) Multi-system repeatability and reproducibility of apparent diffusion coefficient measurement using an ice-water phantom. J Magn Reson Imaging 37:1238–1246
Jerome NP, Papoutsaki MV, Orton MR et al (2016) Development of a temperature-controlled phantom for magnetic resonance quality assurance of diffusion, dynamic, and relaxometry measurements. Med Phys 43:2998–3007
Lee CY, Bennett KM, Debbins JP (2013) Sensitivities of statistical distribution model and diffusion kurtosis model in varying microstructural environments: a Monte Carlo study. J Magn Reson 320:19–26
Ogura A, Hayakawa K, Miyati T, Maeda F (2011) Imaging parameter effects in apparent diffusion coefficient determination of magnetic resonance imaging. Eur J Radiol 77:185–188
Lyng H, Haraldseth O, Rofstad EK (2000) Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med 43:828–836
Szafer A, Jianhui Z et al (1995) Theoretical model for water diffusion in tissues. Magn Reson Med 33:697–712
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Lavel-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Author information
Authors and Affiliations
Contributions
Study conception and design: HY. Acquisition of data: RM, HY, RM, KK, and YY. Analysis and interpretation of data: RM, HY, and MK. Drafting of manuscript: RM. Critical revision: HY, TK, KS, and YY.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mikayama, R., Yabuuchi, H., Matsumoto, R. et al. Development of a new phantom simulating extracellular space of tumor cell growth and cell edema for diffusion-weighted magnetic resonance imaging. Magn Reson Mater Phy 33, 507–513 (2020). https://doi.org/10.1007/s10334-019-00823-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-019-00823-6