Abstract
Aims/hypothesis
Sodium–glucose cotransporter 2 (SGLT2) inhibitors, which prevent the renal reabsorption of glucose, decrease blood glucose levels in an insulin-independent manner. We previously reported creating a mouse model of systemic inhibition of target receptors for both insulin and IGF-1 by treating animals with OSI-906, a dual insulin/IGF-1 receptor inhibitor, for 7 days. The OSI-906-treated mice exhibited an increased beta cell mass, hepatic steatosis and adipose tissue atrophy, accompanied by hyperglycaemia and hyperinsulinaemia. In the present study, we investigated the effects of an SGLT2 inhibitor, luseogliflozin, on these changes in OSI-906-treated mice.
Methods
We treated C57BL/6J male mice either with vehicle, luseogliflozin, OSI-906 or OSI-906 plus luseogliflozin for 7 days, and phenotyping was performed to determine beta cell mass and proliferation. Subsequently, we tested whether serum-derived factors have an effect on beta cell proliferation in genetically engineered beta cells, mouse islets or human islets.
Results
SGLT2 inhibition with luseogliflozin significantly ameliorated hyperglycaemia, but not hyperinsulinaemia, in the OSI-906-treated mice. Liver steatosis and adipose tissue atrophy induced by OSI-906 were not altered by treatment with luseogliflozin. Beta cell mass and proliferation were further increased by SGLT2 inhibition with luseogliflozin in the OSI-906-treated mice. Luseogliflozin upregulated gene expression related to the forkhead box M1 (FoxM1)/polo-like kinase 1 (PLK1)/centromere protein A (CENP-A) pathway in the islets of OSI-906-treated mice. The increase in beta cell proliferation was recapitulated in a co-culture of Irs2 knockout and Insr/IR knockout (βIRKO) beta cells with serum from both luseogliflozin- and OSI-906-treated mice, but not after SGLT2 inhibition in beta cells. Circulating factors in both luseogliflozin- and OSI-906-treated mice promoted beta cell proliferation in both mouse islets and cadaveric human islets.
Conclusions/interpretation
These results suggest that luseogliflozin can increase beta cell proliferation through the activation of the FoxM1/PLK1/CENP-A pathway via humoral factors that act in an insulin/IGF-1 receptor-independent manner.
Similar content being viewed by others

Introduction
Growth factor signalling mediated by activating the insulin receptor (IR) and the IGF-1 receptor (IGF1R) contributes to cell growth, survival and ageing and is involved in the pathogenesis of cancer and metabolic diseases such as diabetes [1, 2]. IR/IGF1R signalling plays a crucial role in the adaptive beta cell proliferation observed in chronic insulin resistance models [3, 4]. The forkhead box M1 (FoxM1)/polo-like kinase 1 (PLK1)/centromere protein A (CENP-A) pathway plays a central role in compensatory beta cell proliferation in response to various metabolic demands including ageing, pregnancy, high-fat diet-induced obesity, acute insulin resistance induced by S961, or neuronal regulation [5, 6]. OSI-906 (linsitinib) is an orally bioavailable dual IR/IGF1R inhibitor [7]. We previously reported that beta cell mass and beta cell proliferation were significantly upregulated after daily administration of OSI-906 for 1 week in mice [8]. Lipodystrophy and liver steatosis were also observed after the administration of OSI-906 for 7 days in mice [9]. Recently, we also reported that the increase in beta cell proliferation seemed to be mediated by cyclin A2/B1/B2, but not by cyclin D1/D2 [9]. Since OSI-906 completely blunts the insulin/IGF1 pathway in beta cells [10], OSI-906-treated mice might serve as a good model for exploring signalling pathways that act independently of IR/IGF1R signalling and are involved in beta cell compensation.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors lower blood glucose levels by inhibiting the reabsorption of glucose from urine in the proximal renal tubules [11]. SGLT2 inhibitors trigger multiple mechanisms that could protect against renal or cardiovascular diseases beyond glucose-lowering effects [12]. These mechanisms have been shown to preserve beta cell mass and function in rodent models [13,14,15,16] and to directly or indirectly improve beta cell function in individuals with type 2 diabetes [17]. Luseogliflozin is a selective potent SGLT2 inhibitor [18] and recent studies have shown that it preserves beta cell mass in obese diabetic db/db mice by enhancing beta cell proliferation or survival [19]. However, the effects of SGLT2 inhibition on beta cell homeostasis remain unclear. In the present study, we investigated the effects of luseogliflozin on the regulation of pancreatic beta cell mass in OSI-906 treated mice.
Methods
Animals and animal care
C57BL/6J male mice (CLEA Japan, Tokyo, Japan) aged 8 weeks old were fed standard chow (Oriental Yeast, Tokyo, Japan) and allowed free access to food and water at room temperature (25°C) under a 12 h light/dark cycle. This study was conducted after the approval of the Yokohama City University Institutional Animal Care and Use Committee (IACUC) (Permit Number: F-A-14-041) and in accordance with the guidelines of the Animal Care Committee of Yokohama City University.
Drug treatments
OSI-906 (linsitinib, #HY-10191) was purchased from MedChem Express (Monmouth Junction, NJ, USA). Luseogliflozin was provided by Taisho Pharmaceutical Co (Tokyo, Japan). The 8-week-old mice were given 10 μl/g weight of either the vehicle (30% [wt/vol.] Solutol HS-15; BASF, Ludwigshafen am Rhein, Germany) or OSI-906 (45 mg/kg) by gavage for 7 days, as previously described [9], 30 min after the oral administration of 10 μl/g weight of either water or luseogliflozin (10 mg/kg/daily, oral gavage) for 7 days between 08:00 and 09:00 hours.
Measurements of biochemical variables
Serum insulin, NEFA, total cholesterol and triacylglycerol levels were assayed as previously described [9]. Samples were collected 4 h after the last OSI-906 administration on day 7. Serum insulin levels were also assayed at 8 or 24 h after a single administration of OSI-906 (45 mg/kg). Blood glucose levels were checked using Glutest Neo Super (Sanwa Chemical Co., Tokyo, Japan) just before and 4 h after the administration of OSI-906 or vehicle.
Immunoblots
The liver was collected at 8 or 24 h after administration of OSI-906 (45 mg/kg). The proteins in tissue samples were extracted using T-PER Tissue Protein Extraction Reagent (with proteases and phosphatase inhibitors) (Thermo Scientific, Waltham, MA, USA). The extracts were then subjected to immunoblotting with antibodies to p-IRβ–IGF1Rβ (Tyr1150/1151, Tyr1135/1136) (#3024, 1/1000), IR (#3015, 1/1000), p-Akt (Ser473) (#9271, 1/1000) and Akt (#9272, 1/1000) (all from Cell Signaling Technology, Danvers, MA, USA). Densitometry was performed using ImageJ software (https://imagej.nih.gov/ij/).
Histological analysis
Mice were injected intraperitoneally with BrdU (100 mg/kg; Nacalai Tesque, Kyoto, Japan); 5 h later, the pancreases were harvested for histological analyses. The dissected pancreases were processed and immunostained with antibodies to insulin (Santa Cruz, TX, USA) and BrdU (Dako, Tokyo, Japan). The beta cell mass and number of BrdU-positive cells were analysed as described previously [20]. All the images were acquired using a BZ-9000 microscope (Keyence, Osaka, Japan) or a FluoView FV1000-D confocal laser scanning microscope (Olympus, Tokyo, Japan). The per cent area of the pancreatic tissue occupied by beta cells was calculated using BIOREVO software (Keyence). In the BrdU immunostaining experiment, approximately 50 islets were analysed using WinROOF software (Mitani, Tokyo, Japan) to assess the proportion of immunostained nuclei among the insulin-positive cells in each mouse. Liver and adipose tissue samples were formalin-fixed, embedded in paraffin, sectioned and stained with haematoxylin and eosin. The white adipocyte areas were measured for 1000 or more cells per mouse in each of the groups using BIOREVO software.
RNA isolation and quantitative RT-PCR
Islets were isolated from 8-week-old wild-type C57BL/6J male mice using the intraductal collagenase technique [21]. Isolated islets were handpicked and the total RNA was extracted using RNeasy Mini Kit (Qiagen, Germantown, MD, USA). One milligram of RNA was reverse-transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA). Quantitative PCR was performed using an ABI 7900HT system with SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). β-Actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as internal controls. The primers described in electronic supplementary material (ESM) Table 1 were used for amplification.
Cell and mouse islet cultures
Beta cell lines from control, Irs1 knockout (IRS1KO), Irs2 knockout (IRS2KO), or beta cell-specific Insr knockout (βIRKO) mice were generated as described previously [22]. The cell lines were obtained from mice with the original 129Sv/C57Bl hybrid background, which were bred with mice expressing the SV40 T antigen (RIP1-Tag2) via the beta cell specific insulin promoter. Mycoplasma contamination was not detected by 16S rRNA-based mycoplasma group-specific PCR. The control, IRS1KO or IRS2KO cells were used between passages 11 and 21, while the βIRKO cells were used between passages 7 and 17. The cells were maintained in DMEM media containing 25 mmol/l glucose, supplemented with 10% FBS (vol./vol.). The experiments were performed using 80–90% confluent cells. The cells were treated with 200 nmol/l OSI-906 (IR/IGF1R dual inhibitor, MedChem Express), 100 nmol/l luseogliflozin (IC50 = 2.26 nmol/l for SGLT2), or serum from mice treated with vehicle (control), vehicle + luseogliflozin (Luse), OSI-906 or OSI-906 plus luseogliflozin (OSI-906+Luse) (20% vol./vol.). Isolated islets were handpicked and cultured in RPMI 1640 media containing 5.6 mmol/l or 11.1 mmol/l glucose and 20% serum from control, Luse-, OSI-906- or OSI-906+Luse-treated mice. The sera were obtained from mice at day 7 of treatment in each group.
Human islets
Human islets were obtained from Clinical Islet Laboratory and Clinical Islet Transplant Program of University of Alberta or the Alberta Diabetes Institute Islet Core of the University of Alberta. All studies and protocols used were approved by Yokohama City University Ethics Board (approval B171100025) and the Joslin Diabetes Center’s Committee on Human Studies (approval CHS#5–05). Details of human islets are described in the ESM Human Islet Checklist. Human islets were handpicked and cultured in Miami Media #1A (Cellgro, Mediatech, Manassas, VA, USA). For the stimulation with serum, the islets were cultured in Final Wash/Culture Medium (Cellgro) containing 20% serum (vol./vol.) from control, Luse-, OSI-906- or OSI-906+Luse-treated mice treated for 48 h in the presence of 5-ethynyl-2-deoxyuridine (EdU). The sera were collected from mice at day 7 of treatment. The islets were embedded in agarose and used for immunostaining studies.
Analysis of cell proliferation
For a modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, cells were plated in 96-well plates (104 cells in each well). The cell number was determined using the CellTiter 96 Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions. For the EdU incorporation assay, islets were treated with 10 μmol/l EdU (24 h for mouse islets and 48 h for human islets) and stained with anti-insulin antibody (Abcam, Cambridge, MA, USA, ab7842, ×400) and the Click-iT Plus EdU Alexa Fluor 488 Imaging Kit (Thermo Fisher, Waltham, MA, USA). TUNEL staining was performed using the ApopTag Fluorescein In Situ Apoptosis Detection Kit (EMD Millipore, Temecula, CA, USA). The images were taken using a FluoView FV1000-D confocal laser scanning microscope. The proliferating beta cells were measured for 500 or more insulin-positive islet cells per mouse, or 50 or more human islets (containing more than 12,000 beta cells) per donor in each of the groups. For TUNEL staining, at least 10,000 cells per group were analysed.
Replicates and inclusion criteria
For the animal study, two independent cohort studies were performed. For experiments using beta cells or mouse islets, more than three independent experiments were conducted. For the human islets study, three distinct samples for each donor were assessed. All the results were taken from distinct samples. The outcomes were substantiated by each repetition of experiments. The mice with abnormality of teeth or human islets with less than 50% viability were omitted from the study.
Statistical analyses
All the data are expressed as the mean ± SEM and were tested for a normal distribution using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA, https://www.graphpad.com/scientific-software/prism/) and SPSS software (IBM, Armonk, NY, USA, https://www.ibm.com/analytics/spss-statistics-software). The statistical significance of the differences was calculated using two-tailed Student’s t test or a one-way ANOVA followed by the Tukey–Kramer post hoc test. Differences with p values of <0.05 (*) or <0.01 (**) were considered significant. Randomisation and blinding were not carried out.
Results
Luseogliflozin ameliorated hyperglycaemia induced by OSI-906
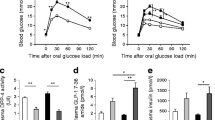
In this study, we administered OSI-906 to mice at a dose of 45 mg/kg, which is known to inhibit IR and IGF1R in the liver and white adipose tissue [9]. We performed a 7 day study followed by systematic phenotyping to compare mice treated with vehicle (control), Luse, OSI-906 or OSI-906+Luse (Fig. 1a). Hyperinsulinaemia and the reduction in the phosphorylation of IR/IGF1R or Akt in the liver was sustained until 8 or 24 h after an administration of OSI-906 at 45 mg/kg, although hyperglycaemia had disappeared by 24 h (ESM Fig. 1a–c). No differences were observed in body weight between the OSI-906-treated mice compared with control or OSI-906+Luse groups, throughout the study (Fig. 1b). The OSI-906+Luse group exhibited a significant reduction in body weight from day 3 to day 4, compared with the control group; however, by day 7 the body weights were similar between the two groups (Fig. 1b). The OSI-906 group showed hyperglycaemia at 4 h after each administration and normoglycaemia at 24 h (just before the next administration), and luseogliflozin treatment significantly ameliorated the OSI-906-induced hyperglycaemia (Fig. 1c). Significant increases in plasma insulin (Fig. 1d), NEFA (Fig. 1e) and triacylglycerol (Fig. 1f) levels were observed at day 7 in the OSI-906 group, while the serum total cholesterol (Fig. 1g) remained unaltered; these changes were comparable between the OSI-906 and OSI-906+Luse groups.
Luseogliflozin improved hyperglycaemia induced by OSI-906. (a) Experimental protocol (n = 6). (b) Body weight gain in the four groups over 7 days of treatment, and shown in separate graphs on days 3 and 4; data represent the mean ± SEM. *p < 0.05, OSI-906+Luse vs control by one-way ANOVA, followed by the Tukey–Kramer post hoc test (n = 6 per group). (c) Blood glucose levels over 7 days of treatment, with AUC for blood glucose. Measurements were taken twice a day, just before and 4 h after the treatment; data represent the mean ± SEM. **p < 0.01, OSI-906 vs control, Luse and OSI-906+Luse groups, or as shown; ††p < 0.01, OSI-906+Luse vs both control and Luse; ‡p < 0.05, OSI-906+Luse vs Luse; by one-way ANOVA, followed by the Tukey–Kramer post hoc test (n = 6 per group). (d) Serum insulin, (e) NEFA, (f) triacylglycerol (TG) and (g) total cholesterol (TChol) levels. (d-g) The measurements were performed at day 7 of treatment. Data in (d–g) represent the mean ± SEM. *p < 0.05, **p < 0.01 as shown, by one-way ANOVA, followed by the Tukey–Kramer post hoc test (n = 6 per group)
Liver steatosis and adipose tissue atrophy were not ameliorated by luseogliflozin in OSI-906-treated mice
The administration of OSI-906 increased the liver size, accompanied by an increased liver weight, and induced hepatic steatosis by day 7 (ESM Fig. 2a–c); these values were not altered in the OSI-906+Luse group (ESM Fig. 2a–c). Luseogliflozin also had no significant effects on the increase in hepatic triacylglycerol content in OSI-906-treated mice observed on day 7 (ESM Fig. 2d). The atrophic changes in visceral adipose tissue induced by OSI-906 were confirmed using computer tomography imaging (ESM Fig. 3a) and by histological analysis of adipocyte size in epididymal fat (ESM Fig. 3b). The administration of luseogliflozin to the OSI-906-treated mice did not improve the fat atrophy or the reduced adipocyte size (ESM Fig. 3a, b). Thus, liver steatosis and adipose tissue atrophy by IR and IGF1R inhibition were not ameliorated in the OSI-906+Luse group, even though the hyperglycaemia was corrected in these mice.
Luseogliflozin further increased beta cell proliferation in OSI-906-treated mice
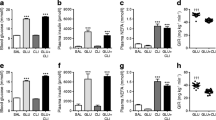
The pancreas weights showed no significant changes after treatment with OSI-906 or luseogliflozin (ESM Fig. 4). The beta cell mass and beta cell proliferation were comparable between the control and Luse groups (Fig. 2a, b). The OSI-906 group exhibited an increase in both the beta cell mass and beta cell proliferation on day 7 (Fig. 2a, b). The OSI-906+Luse group had a significantly greater beta cell mass than the OSI-906 group (Fig. 2a) consistent with a significant increase in the beta cell proliferation in the OSI-906+Luse-treated animals (Fig. 2b). Interestingly, islets from the OSI-906 group showed a significant upregulation of Foxm1 (Fig. 2c), Cenpa (Fig. 2d) and Plk1 (Fig. 2e) expression, compared with the levels observed in islets from control mice. An increased expression of downstream genes of the FoxM1/PLK1/CENP-A pathway, such as Ccnb1 (Fig. 2f), Ccnb2 (Fig. 2g), Cdk1 (Fig. 2h) and Survivin (also known as Birc5; Fig. 2i), was observed in islets from the OSI-906 group, compared with the control group, whereas the expression of Irs2 (Fig. 2j), Ccnd1 (Fig. 2k) and Ccnd2 (Fig. 2l), which are downstream molecules of insulin signalling in beta cells, were unchanged. Treatment with luseogliflozin further augmented the gene expression of Foxm1, Cenpa, Plk1, Ccnb1, Ccnb2 and Cdk1 in the OSI-906 treated mice (Fig. 2c–h). However, the expression of Irs2, Ccnd1 and Ccnd2 were comparable between the OSI-906 and OSI-906+Luse groups (Fig. 2j–l). These results indicated that insulin-independent signals induced by luseogliflozin enhanced the FoxM1/PLK1/CENP-A pathway, increasing beta cell proliferation in response to acute insulin resistance due to IR and IGF1R inhibition.
Luseogliflozin augmented beta cell proliferation in OSI-906-treated mice. (a) Pancreas sections, treated as indicated, were immunostained for insulin (brown; representative micrograph shown) and used to measure beta cell mass; scale bar, 300 μm (n = 6 per group). (b) Pancreatic sections, treated as indicated, were stained with DAPI (blue), antibodies to insulin (green) and BrdU (red) (representative micrograph shown) and used to calculate the percentage of BrdU+ beta cells in the islets; white arrows indicate BrdU-incorporating proliferating beta cells; scale bar, 50 μm (n = 6 per group). (c–l) Relative mRNA expression levels of Foxm1 (c), Cenpa (d), Plk1 (e), Ccnb1 (f), Ccnb2 (g), Cdk1 (h), Survivin (i), Irs2 (j), Ccnd1 (k) and Ccnd2 (l), normalised to β-actin and plotted as fold change vs control, in the islets (n = 6 per group). All data represent the mean ± SEM. *p < 0.05, **p < 0.01 as shown, by one-way ANOVA, followed by the Tukey–Kramer post hoc test
Humoral factors mediate luseogliflozin-induced beta cell proliferation in OSI-906-treated mice independent of insulin signalling
βIRKO (beta cell-specific Insr/IR knockout) beta cells possess an impaired proliferation capacity, compared with control beta cells [22]. IRS2 plays a pivotal role in glucose-induced beta cell proliferation [23]. The evaluation of an MTT assay revealed that the control, βIRKO, IRS1KO and IRS2KO beta cells all demonstrated similar cell viabilities even in the presence of OSI-906 or luseogliflozin (Fig. 3a–d), suggesting that neither OSI-906 nor luseogliflozin has direct effects on cell proliferation. Next, treating cells with serum from the OSI-906 or OSI-906+Luse group led to significantly increased cell viability in the latter group in the control, IRS1KO, IRS2KO, as well as the IR-deficient βIRKO beta cells (Fig. 3e–h).
Humoral factors in both luseogliflozin- and OSI-906-treated mice evoked beta cell replication independent of insulin signalling. (a–d) MTT assay (absorbance at 570 nm) in control, βIRKO, IRS1KO and IRS2KO beta cells in the presence/absence of OSI-906 and/or luseogliflozin (n = 6 per group). (e–h) MTT assay (absorbance at 570 nm) in indicated cells in the presence of medium containing 20% serum from OSI-906- or OSI-906+Luse-treated mice. The sera were collected from mice at day 7 of treatment (n = 6 per group). All data represent the mean ± SEM. *p< 0.05 by two-tailed Student’s t test
Since the blood glucose level was corrected by luseogliflozin in the OSI-906-treated mice, we cultured isolated mouse islets with sera under normal (5.6 mmol/l) or high (11.1 mmol/l) glucose conditions. High glucose stimulation significantly enhanced the incorporation of EdU in proliferating beta cells in the islets (Fig. 4a). Notably, serum from the OSI-906+Luse group significantly potentiated beta cell proliferation, compared with serum from the OSI-906 group, under both normal and high glucose conditions (Fig. 4a). In islets co-cultured with serum from the OSI-906+Luse group, the gene expression levels of Foxm1, Cenpa, Plk1 and Ccnb1 were significantly upregulated (Fig. 4b). These results suggested that luseogliflozin induces beta cell proliferation via a humoral factor-mediated FoxM1/PLK1/CENP-A pathway that acts independently of insulin signalling.
Circulating factors in both luseogliflozin- and OSI-906-treated mice increase beta cell proliferation in mouse islets via the FoxM1/PLK1/CENP-A pathway. (a) The sera were collected from mice at day 7 of treatment. Mouse islets, treated with 20% serum from OSI-906- or OSI-906+Luse-treated mice, under 5.6 or 11.1 mmol/l glucose-containing media for 24 h, were stained with DAPI (blue), antibodies to insulin (red) and EdU (green) (representative micrograph shown) and used to calculate the percentage of EdU+ beta cells in the cultured islets; white arrows indicate EdU-incorporating proliferating beta cells; scale bar, 50 μm (n = 6 per group). (b) The sera were collected from mice at day 7 of treatment. Relative mRNA expression levels of Foxm1, Cenpa, Plk1, Ccnb1 and Ccnb2, normalised to β-actin and plotted as fold change vs the +20% OSI-906 serum group, in the islets cultured with 20% serum from OSI-906- or OSI-906+Luse-treated mice, under 11.1 mmol/l glucose-containing media for 24 h (n = 6 per group). All data represent the mean ± SEM. *p < 0.05 as shown, by one-way ANOVA, followed by the Tukey–Kramer post hoc test
The cell viability of control beta cells, the beta cell proliferation in mouse islets, and the expression of Foxm1, Cenpa, Plk1, Ccnb1 and Ccnb2 in mouse islets, after the treatment with serum, did not differ between control and Luse-treated groups (ESM Fig. 5a–c). Therefore, the OSI-906-induced metabolic alterations such as hyperglycaemia, hyperinsulinaemia, or tissue insulin resistance are a potential prerequisite for induction of circulating mitogenic factors for beta cells following SGLT2 inhibition. SerpinB1 and IGFBP1 have been reported as liver-derived circulating factors with the ability to promote beta cell proliferation in response to insulin resistance [24, 25]. The expression levels of Serpinb1 (also known as Serpinb1a) and Igfbp1 were significantly upregulated in the livers of the OSI-906 group (ESM Fig. 6a, b). However, the administration of luseogliflozin did not further alter the hepatic expression of these growth factors in OSI-906-treated mice (ESM Fig. 6a, b), suggesting that SerpinB1 and IGFBP1 are unlikely to be primary causal factors promoting beta cell proliferation induced by luseogliflozin.
Circulating factors induced by luseogliflozin in OSI-906-treated mice promoted beta cell proliferation in human islets
The proliferative ability of putative circulating factors in the OSI-906+Luse group was also tested in human beta cells from six donors without diabetes (donor characteristics are described in the ESM Human Islet Checklist). EdU-incorporated proliferating human beta cells significantly increased in islets cultured in media containing serum from the OSI-906+Luse group, compared with that from the OSI-906 group (Fig. 5a) and the proportion of proliferating human beta cells was similar to that in the previous report [24]. The analysis of gene expression data showed that PLK1 and CCNB1 were significantly increased in human islets co-cultured with serum from the OSI-906+Luse group, compared with human islets treated with serum from the OSI-906 group (Fig. 5b). The expression of FOXM1 (p = 0.07), CENPA (p = 0.08) and CCNB2 (p = 0.12) were not significantly different between the OSI-906 and OSI-906+Luse serum-treated islets. However, the proportion of TUNEL-positive apoptotic beta cells was comparable between the two groups (ESM Fig. 7). Thus, circulating factors induced by luseogliflozin in OSI-906-treated mice can enhance the proliferative capacity of human beta cells, probably as a result of activation of FoxM1/PLK/CENP-A pathway.
Humoral factors in both luseogliflozin- and OSI-906-treated mice increased beta cell proliferation in human islets. (a) The sera were collected from mice at day 7 of treatment. Human islets from non-diabetic donors, treated with 20% serum from OSI-906- or OSI-906+Luse-treated mice, for 24 h, were stained with DAPI (blue), antibodies to insulin (red) and EdU (green) (representative micrograph shown) and used to calculate the percentage of EdU+ beta cells in the cultured islets; white arrows indicate EdU-incorporating proliferating beta cells; scale bar, 50 μm (n = 6 per group). (b) The sera were collected from mice at day 7 of treatment. Relative mRNA expression levels of FOXM1, CENPA, PLK1, CCNB1 and CCNB2, normalised to GAPDH and plotted as fold change vs the +20% OSI-906 serum group, in human islets cultured with 20% serum from OSI-906- or OSI-906+Luse-treated mice, for 24 h (n = 5 per group). All data represent the mean ± SEM. *p < 0.05 as shown, by two-tailed Student’s t test
Discussion
In this study, we report the effects of an SGLT2 inhibitor, luseogliflozin, on hepatic steatosis, visceral fat atrophy and beta cell proliferation in OSI-906-treated mice. SGLT2 inhibition with luseogliflozin improved glucose levels and increased the beta cell proliferation, while the hyperinsulinaemia, fatty liver and lipoatrophy were unaffected. We further investigated how luseogliflozin facilitates cell replication in beta cells using IRS1KO, IRS2KO and βIRKO beta cells or mouse and human islets.
The physiological mechanisms underlying the compensatory beta cell growth in response to insulin resistance are not yet fully understood. Insulin signalling, including via IRS2, mammalian target of rapamycin (mTOR) and cyclin D2, a downstream factor of insulin signalling, have been demonstrated to be required for beta cell expansion in response to a high-fat diet (HFD) [26,27,28,29]. Interestingly, we have previously reported that GLP-1 is able to enhance beta cell proliferation in βIRKO mice [30]. Furthermore, short-term HFD-feeding induces beta cell proliferation without increased expression of cyclin D2 [31]. Intriguingly, a recent study, albeit using a partial IR knockdown model, reported that glucose induces beta cell proliferation by modulating IRS2, mTOR and cyclin D2 signalling independent of activating the IR [23]. One interpretation of these collective findings is that factors acting independent of IR/IGF1R signalling can promote short-term compensatory beta cell growth.
We previously reported that acute inhibition of IR/IGF1R signalling is able to induce hyperglycaemia, hyperinsulinaemia and increased beta cell mass and proliferation in OSI-906-treated mice [8, 9]. These results suggested that the beta cell proliferation was mediated by an insulin signalling-independent pathway, as no change in the cyclin D2 expression was observed in the mouse islets [9]. Hence, this model of acute inhibition of IR/IGF1R signalling is useful for investigating potential insulin signalling-independent expansion of beta cell mass. In the present study, both the OSI-906 and the OSI-906+Luse groups showed increased beta cell proliferation. Since luseogliflozin improved glucose levels without altering serum insulin levels in the OSI-906-treated mice, sustained hyperglycaemia is unlikely to contribute to the beta cell proliferation observed in the OSI-906-treated mice as suggested by other studies [26].
The OSI-906+Luse-treated group exhibited improved glycaemic control and augmented beta cell mass and proliferation, compared with the OSI-906 group. SGLT2 knockout in db/db mice preserved beta cell function, in accordance with an increase in the beta cell mass and a reduction in beta cell apoptosis [32]. SGLT2 inhibitors have been shown to increase beta cell proliferation and to decrease beta cell apoptosis in rodent models of type 1 and type 2 diabetes [13,14,15,16, 19]; thus, the amelioration of glucotoxicity by SGLT2 inhibitors is likely to contribute to beta cell protection. Because beta cell proliferation in isolated islets was induced by serum from OSI-906+Luse mice independent of the glucose levels, signals activated by glucose are dispensable for beta cell proliferation induced by SGLT2 inhibition in this study.
Previous studies have reported that treatment with SGLT2 inhibitors can ameliorate hepatic steatosis in diet-induced obese mice and in individuals with type 2 diabetes and non-alcoholic fatty liver disease [33, 34]. In the present study, however, the OSI-906+Luse group showed no changes in hepatic steatosis and hepatic triacylglycerol content when compared with the OSI-906 group. These results suggest that the improvement in fatty liver induced by SGLT2 inhibition is mediated through a modification of hepatic insulin signalling. OSI-906 also induced lipoatrophy, which is represented by visceral fat atrophy and a reduction in adipocyte size possibly by promotion of lipolysis [9]. Because luseogliflozin did not affect lipoatrophy, the inhibition of insulin signalling by OSI-906 should be unaffected by SGLT2 inhibition in those mice. Hence, luseogliflozin-induced beta cell proliferation probably acts independent of the impact of luseogliflozin on hepatic steatosis and/or fat atrophy.
We recently reported that insulin signalling potentiates the signalling pathway of FoxM1/PLK1/CENP-A to promote beta cell proliferation in response to various metabolic demands [5]. In this study, the FoxM1/PLK1/CENP-A pathway contributed to beta cell proliferation in both the OSI-906 and OSI-906+Luse groups. This finding is consistent with the result that CENP-A is indispensable for beta cell proliferation in mice treated with S961, a peptide that antagonises the IR [5, 35]. Since IRS2KO and βIRKO beta cells both showed an increase in cell replication in response to serum from the OSI-906+Luse group, elevated insulin levels as a result of hyperinsulinaemia are unlikely to be necessary to increase beta cell proliferation in our model. It is also likely that luseogliflozin indirectly increased the beta cell proliferation by humoral circulating factors other than SerpinB1 or IGFBP1 in this study. Glucose stimulation was able to further increase beta cell proliferation in mouse islets treated with serum from the OSI-906+Luse group. Thus, it is possible that activation of signalling proteins in response to hyperglycaemia acts additively to promote beta cell replication in our model. Further investigation is necessary to clarify the target organs and factors involved in the inter-organ regulation of beta cell proliferation by OSI-906 or SGLT2 inhibition. We also observed that circulating factors in serum from OSI-906+Luse mice enhanced beta cell proliferation in human islets. These results were consistent with a previous observation: CENP-A and PLK1 are crucial for adaptive beta cell proliferation in human beta cells [5].
Taken together, the present findings suggest that luseogliflozin increases beta cell proliferation through humoral factors that stimulate an insulin signalling-independent FoxM1/PLK1/CENP-A pathway in OSI-906-treated mice. Insulin/IR-mediated signalling is reportedly impaired in islets from donors with type 2 diabetes, compared with those from donors without diabetes [36, 37]. Therefore, the elucidation of a pathway to enhance beta cell mass independently of insulin signalling represents an attractive therapeutic strategy for type 2 diabetes. Identifying the circulating factors that are regulated by SGLT2 inhibitors and defining how those factors contribute to beta cell proliferation offers a potential approach to compensate for the loss of beta cell mass in diabetes.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- βIRKO:
-
Beta cell-specific Insr knockout
- CENP-A:
-
Centromere protein A
- EdU:
-
5-Ethynyl-2-deoxyuridine
- FoxM1:
-
Forkhead box M1
- IGF1R:
-
IGF-1 receptor
- IR:
-
Insulin receptor
- IRS1KO:
-
Irs1 knockout
- IRS2KO:
-
Irs2 knockout
- Luse:
-
Luseogliflozin (experimental group)
- mTOR:
-
Mammalian target of rapamycin
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PLK1:
-
Polo-like kinase 1
- SGLT2:
-
Sodium–glucose cotransporter 2
References
Djiogue S, Nwabo Kamdje AH, Vecchio L et al (2013) Insulin resistance and cancer: the role of insulin and IGFs. Endocr Relat Cancer 20(1):R1–R17. https://doi.org/10.1530/ERC-12-0324
Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R (2009) Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30(6):586–623. https://doi.org/10.1210/er.2008-0047
Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR (1999) Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96(3):329–339. https://doi.org/10.1016/s0092-8674(00)80546-2
Ueki K, Okada T, Hu J et al (2006) Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat Genet 38(5):583–588. https://doi.org/10.1038/ng1787
Shirakawa J, Fernandez M, Takatani T et al (2017) Insulin signaling regulates the FoxM1/PLK1/CENP-A pathway to promote adaptive pancreatic beta cell proliferation. Cell Metab 25:868–882
Yamamoto J, Imai J, Izumi T et al (2017) Neuronal signals regulate obesity induced beta-cell proliferation by FoxM1 dependent mechanism. Nat Commun 8:1930
Mulvihill MJ, Cooke A, Rosenfeld-Franklin M et al (2009) Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med Chem 1:1153–1171
Shirakawa J, Okuyama T, Yoshida E et al (2014) Effects of the antitumor drug OSI-906, a dual inhibitor of IGF-1 receptor and insulin receptor, on the glycemic control, beta-cell functions, and beta-cell proliferation in male mice. Endocrinology 155(6):2102–2111. https://doi.org/10.1210/en.2013-2032
Tajima K, Shirakawa J, Togashi Y et al (2017) Metabolic recovery of lipodystrophy, liver steatosis, and pancreatic beta cell proliferation after the withdrawal of OSI-906. Sci Rep 7:4119
Mezza T, Shirakawa J, Martinez R, Hu J, Giaccari A, Kulkarni RN (2016) Nuclear export of FoxO1 is associated with ERK signaling in beta-cells lacking insulin receptors. J Biol Chem 291(41):21485–21495. https://doi.org/10.1074/jbc.M116.735738
Tahrani AA, Barnett AH, Bailey CJ (2013) SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol 1(2):140–151. https://doi.org/10.1016/S2213-8587(13)70050-0
Zelniker TA, Braunwald E (2018) Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC State-of-the-Art Review. J Am Coll Cardiol 72(15):1845–1855. https://doi.org/10.1016/j.jacc.2018.06.040
Shimo N, Matsuoka TA, Miyatsuka T et al (2015) Short-term selective alleviation of glucotoxicity and lipotoxicity ameliorates the suppressed expression of key beta-cell factors under diabetic conditions. Biochem Biophys Res Commun 467(4):948–954. https://doi.org/10.1016/j.bbrc.2015.10.038
Cheng ST, Chen L, Li SY, Mayoux E, Leung PS (2016) The effects of empagliflozin, an SGLT2 inhibitor, on pancreatic β-cell mass and glucose homeostasis in type 1 diabetes. PLoS One 11:e0147391
Okauchi S, Shimoda M, Obata A et al (2016) Protective effects of SGLT2 inhibitor luseogliflozin on pancreatic beta-cells in obese type 2 diabetic db/db mice. Biochem Biophys Res Commun 470(3):772–782. https://doi.org/10.1016/j.bbrc.2015.10.109
Macdonald FR, Peel JE, Jones HB et al (2010) The novel sodium glucose transporter 2 inhibitor dapagliflozin sustains pancreatic function and preserves islet morphology in obese, diabetic rats. Diabetes Obes Metab 12(11):1004–1012. https://doi.org/10.1111/j.1463-1326.2010.01291.x
Polidori D, Mari A, Ferrannini E (2014) Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves model-based indices of beta cell function in patients with type 2 diabetes. Diabetologia 57:891–901
Kakinuma H, Oi T, Hashimoto-Tsuchiya Y et al (2010) (1S)-1,5-anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-D-glucito l (TS-071) is a potent, selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment. J Med Chem 53(8):3247–3261. https://doi.org/10.1021/jm901893x
Kimura T, Obata A, Shimoda M et al (2018) Protective effects of the SGLT2 inhibitor luseogliflozin on pancreatic β-cells in db/db mice: the earlier and longer, the better. Diabetes Obes Metab 20(10):2442–2457. https://doi.org/10.1111/dom.13400
Togashi Y, Shirakawa J, Orime K et al (2014) β-Cell proliferation after a partial pancreatectomy is independent of IRS-2 in mice. Endocrinology 155:1643–1652
Shirakawa J, Togashi Y, Sakamoto E et al (2013) Glucokinase activation ameliorates ER stress-induced apoptosis in pancreatic beta-cells. Diabetes 62(10):3448–3458. https://doi.org/10.2337/db13-0052
Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN (2009) Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol 29(11):3219–3228. https://doi.org/10.1128/MCB.01489-08
Stamateris RE, Sharma RB, Kong Y et al (2016) Glucose induces mouse β-cell proliferation via IRS2, MTOR, and Cyclin D2 but not the insulin receptor. Diabetes 65(4):981–995. https://doi.org/10.2337/db15-0529
El Ouaamari A, Dirice E, Gedeon N et al (2016) Serpinb1 promotes pancreatic β cell proliferation. Cell Metab 23(1):194–205. https://doi.org/10.1016/j.cmet.2015.12.001
Lu J, Liu KC, Schulz N et al (2016) IGFBP1 increases β-cell regeneration by promoting α- to β-cell transdifferentiation. EMBO J 35(18):2026–2044. https://doi.org/10.15252/embj.201592903
Okada T, Liew CW, Hu J et al (2007) Insulin receptors in β-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci U S A 104:8977–8982
Terauchi Y, Takamoto I, Kubota N et al (2007) Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest 117(1):246–257. https://doi.org/10.1172/JCI17645
Sachdeva MM, Claiborn KC, Khoo C et al (2009) Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc Natl Acad Sci U S A 106(45):19090–19095. https://doi.org/10.1073/pnas.0904849106
Lakshmipathi J, Alvarez-Perez JC, Rosselot C et al (2016) PKCζ Is essential for pancreatic β-cell replication during insulin resistance by regulating mTOR and Cyclin-D2. Diabetes 65(5):1283–1296. https://doi.org/10.2337/db15-1398
Kawamori D, Shirakawa J, Liew CW et al (2017) GLP-1 signalling compensates for impaired insulin signalling in regulating beta cell proliferation in βIRKO mice. Diabetologia 60(8):1442–1453. https://doi.org/10.1007/s00125-017-4303-6
Mosser RE, Maulis MF, Moulle VS et al (2015) High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am J Physiol Endocrinol Metab 308(7):E573–E582. https://doi.org/10.1152/ajpendo.00460.2014
Jurczak MJ, Lee HY, Birkenfeld AL et al (2011) SGLT2 deletion improves glucose homeostasis and preserves pancreatic β-cell function. Diabetes 60(3):890–898. https://doi.org/10.2337/db10-1328
Kuchay MS, Krishan S, Mishra SK et al (2018) Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care 41(8):1801–1808. https://doi.org/10.2337/dc18-0165
Xu L, Nagata N, Nagashimada M et al (2017) SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine 20:137–149. https://doi.org/10.1016/j.ebiom.2017.05.028
Schaffer L, Brand CL, Hansen BF et al (2008) A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun 376(2):380–383. https://doi.org/10.1016/j.bbrc.2008.08.151
Folli F, Okada T, Perego C et al (2011) Altered insulin receptor signalling and β-cell cycle dynamics in type 2 diabetes mellitus. PLoS One 6:e28050
Gunton JE, Kulkarni RN, Yim S et al (2005) Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122(3):337–349. https://doi.org/10.1016/j.cell.2005.05.027
Acknowledgements
We thank P. MacDonald (Alberta Diabetes Institute, University of Alberta) for providing human islets from Alberta Diabetes Institute IsletCore. We thank E. Sakamoto and M. Kaji (Yokohama City University) for their excellent technical assistance and animal care and M. Katayama (Yokohama City University) for her secretarial assistance. We also thank Taisho Pharmaceutical Co (Tokyo, Japan) for providing the luseogliflozin.
Contribution statement
JS, KT, RNK and YTe conceived the design. JS, KT, TO, MK, YTo, DFDJ and GB researched the data. TK and AMJS contributed to human islet preparation, analysis of data, and interpretation of data. JS, KT, RNK and YTe wrote the manuscript, contributed to the discussion and reviewed and edited the manuscript. TO, MK, YTo, DFDJ, GB, TK and AMJS revised the manuscript. All authors approved the final version of the manuscript. JS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (B) 16H05329 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (to YTe) and a Grant-in-Aid for Young Scientists (B) 18K16240 from MEXT of Japan, Junior Scientist Development Grant provided by Novo Nordisk Pharma, Kanae Foundation for the Promotion of Medical Science, Suzuken Memorial Foundation, Japan Foundation for Applied Enzymology (FFDR), Ono Medical Research Foundation, Kamome Memorial Foundation of Yokohama City University, Takeda Science Foundation, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, AMED-ASTAR Joint Grant Call for Strategic International Collaborative Research Program (SICORP), and a grant from the Japan IDDM network (to JS). RNK acknowledges support from NIH RO1 DK67536, RO1 DK117639 and DK P036836. The study funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM
(PDF 966 kb)
Rights and permissions
About this article
Cite this article
Shirakawa, J., Tajima, K., Okuyama, T. et al. Luseogliflozin increases beta cell proliferation through humoral factors that activate an insulin receptor- and IGF-1 receptor-independent pathway. Diabetologia 63, 577–587 (2020). https://doi.org/10.1007/s00125-019-05071-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-019-05071-w