Abstract
Molybdenum disulfide quantum dots (MoS2 QDs) were chosen as a functional two-dimensional material to improve the separation performance of a traditional C18 column. In this work, MoS2 QDs were synthesized by the combination of sonication and solvothermal treatment of bulk MoS2. The prepared MoS2 QDs were characterized by transmission electron microscope (TEM), Zeta potential measurement, UV-visible absorption and fluorescence spectroscopy. Then, a novel MoS2 QDs embedded C18 (Sil-MoS2-C18) stationary phase was prepared for performing mixed-mode liquid chromatography. The results of elemental analysis (EA), thermogravimetric analysis (TGA), Fourier transform infrared spectrometry (FT-IR), X-ray photoelectron spectroscopy (XPS), scanning electron microscope (SEM) and Brunauer-Emmett-Teller (BET) measurements indicated the stationary phase was prepared successfully. Five types of compounds including alkylbenzenes, polycyclic aromatic hydrocarbons (PAHs), nucleosides and nucleobases, anilines and flavonoids were utilized to evaluate reversed phase, weak cation exchange and hydrophilic interaction of the new column. To a certain extent, the column could achieve separation for different properties of samples on one column, with less organic solvent and shorter time than conventional alkyl and amino columns. Furthermore, the mechanism for separation was studied by investigating effects of mobile phase composition and pH on retentions. In summary, the Sil-MoS2-C18 stationary phase was deemed able to serve the performance of various types of phases, which revealed the prepared mixed-mode column could be potentially applied for the analysis of complex samples.

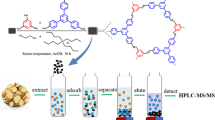
Graphical abstract









Similar content being viewed by others
References
Chen H, Wen X, Zhang J, Wu T, Gong Y, Zhang X, et al. Ultrafast formation of interlayer hot excitons in atomically thin MoS2/WS2 heterostructures. Nat. Commun. 2016;7:12512.
Behura S, Nguyen P, Che S, Debbarma R, Berry V. Large-area, transfer-free, oxide-assisted synthesis of hexagonal boron nitride films and their heterostructures with MoS2 and WS2. J. Am. Chem. Soc. 2015;137:13060–5.
Mohanty B, Ghorbani-Asl M, Kretschmer S, Ghosh A, Guha P, Panda SK, et al. MoS2 quantum dots as efficient catalyst materials for the oxygen evolution reaction. ACS Catal. 2018;8:1683–9.
Er D, Ye H, Frey NC, Kumar H, Lou J. Shenoy VB. Prediction of enhanced catalytic activity for hydrogen evolution reaction in Janus transition metal dichalcogenides Nano Lett. 2018;18:3943–9.
Hu L, Zhang Q, Gan X, Yin W, Fu W. Switchable fluorescence of MoS2 quantum dots: a multifunctional probe for sensing of chromium (VI), ascorbic acid, and alkaline phosphatase activity. Anal Bioanal Chem. 2018;410:7551–7.
Yun Q, Lu Q, Zhang X, Tan C, Zhang H. Three-dimensional architectures constructed from transition-metal dichalcogenide nanomaterials for electrochemical energy storage and conversion. Angew. Chem., Int. Ed. 2018;57:626–46.
Voiry D, Yamaguchi H, Li JW, Silva R, Alves DCB, Fujita T, et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 2013;12:850–5.
Splendiani A, Sun L, Zhang Y, Li T, Kim J, Chim C, et al. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010;10:1271–5.
Xu S, Li D, Wu P. One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015;25:1127–36.
Gu W, Yan Y, Cao X, Zhang C, Ding C, Xian Y. A facile and one-step ethanol-thermal synthesis of MoS2 quantum dots for two-photon fluorescence imaging. J. Mater. Chem. B. 2016;4:27–31.
Dong H, Tang S, Hao Y, Yu H, Dai W, Zhao G, et al. Fluorescent MoS2 quantum dots: ultrasonic preparation, up-conversion and down-conversion bioimaging, and photodynamic therapy. ACS Appl. Mater. Interfaces. 2016;8:3107–14.
Ou G, Fan P, Ke X, Xu Y, Huang K, Wei H, et al. Defective molybdenum sulfide quantum dots as highly active hydrogen evolution electrocatalysts. Nano Res. 2018;11:751–61.
Marqus S, Ahmed H, Ahmed M, Xu C, Rezk AR, Yeo LY. Increasing exfoliation yield in the synthesis of MoS2 quantum dots for optoelectronic and other applications through a continuous multicycle acoustomicrofluidic approach. ACS Appl. Nano Mater. 2018;1:2503–8.
Wang F, Qu X, Liu D, Ding C, Zhang C, Xian Y. Upconversion nanoparticles-MoS2 nanoassembly as a fluorescent turn-on probe for bioimaging of reactive oxygen species in living cells and zebrafish, Sens. Actuators, B. 2018;274:180–7.
Chen J, Li Y, Huang Y, Zhang H, Chen X, Qiu H. Fluorometric dopamine assay based on an energy transfer system composed of aptamer-functionalized MoS2 quantum dots and MoS2 nanosheets. Microchim. Acta. 2019;186:58.
Jia J, Xu F, Wang S, Jiang X, Hou X. Two-dimensional MoS2 nanosheets as a capillary GC stationary phase for highly effective molecular screening. Analyst. 2014;139:3533–6.
Zhang M, Liang X, Jiang S, Qiu H. Preparation and applications of surface-confined ionic-liquid stationary phases for liquid chromatography. Trends Anal. Chem. 2014;53:60–72.
Dai L, Guo N, Liu Y, Shen S, Ge Q, Pan Y. Analysis of the binding sites with NL-101 to amino acids and peptides by HPLC/MS/MS. Chinese Chemical Letters. 2019;30:103–6.
Wen Y, Talebi M, Amos RIJ, Szucs R, Dolan JW, Pohl CA, et al. Retention prediction in reversed phase high performance liquid chromatography using quantitative structure-retention relationships applied to the hydrophobic subtraction model. J. Chromatogr. A. 2018;1541:1–11.
Jandera P, Janás P. Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review, Anal. Chim. Acta. 2017;967:12–32.
Qian K, Yang Z, Zhang F, Yang B, Dasgupta PK. Low-bleed silica-based stationary phase for hydrophilic interaction liquid chromatography. Anal. Chem. 2018;90:8750–5.
Song C, Wang J, Zhao K, Bai Q. Preparation and characterization of a novel dual-retention mechanism mixed-mode stationary phase with PEG 400 and succinic anhydride as ligand for protein separation in WCX and HIC modes. Biomed. Chromatogr. 2013;27:1741–53.
Krzemińska K, Bocian S. The versatility of N, O-dialkylphosphoramidate stationary phase-separations in HILIC, highly aqueous RPLC conditions and purely aqueous mobile phase. Analyst. 2018;143:1217–23.
Wang L, Wei W, Xia Z, Jie X, Xia ZZ. Recent advances in materials for stationary phases of mixed-mode high-performance liquid chromatography, TrAC. Trends Anal. Chem. 2016;80:495–506.
Larry W. McLaughlin, Mixed-mode chromatography of nucleic acids. Chem. Rev. 1989;89:309–19.
Ren X, Zhang K, Gao D, Fu Q, Zeng J, Zhou D, et al. Mixed-mode liquid chromatography with a stationary phase co-functionalized with ionic liquid embedded C18 and an aryl sulfonate group. J. Chromatogr. A. 2018;1564:137–44.
Ray S, Takafuji M, Ihara H. Chromatographic evaluation of a newly designed peptide-silica stationary phase in reverse phase liquid chromatography and hydrophilic interaction liquid chromatography: Mixed mode behavior. J. Chromatogr. A. 2012;1266:43–52.
Mallik AK, Cheah WK, Shingo K, Ejzaki A, Takafuji M, Ihara H. Highly hydrophilic and nonionic poly(2-vinyloxazoline)-grafted silica: a novel organic phase for high-selectivity hydrophilic interaction chromatography. Anal Bioanal Chem. 2014;406:4585–93.
Zhou D, Zeng J, Fu Q, Gao D, Zhang K, Ren X, et al. Preparation and evaluation of a reversed-phase/hydrophilic interaction/ion-exchange mixed-mode chromatographic stationary phase functionalized with dopamine-based dendrimers. J. Chromatogr. A. 2018;1571:165–75.
Ren X, Hu C, Gao D, Fu Q, Zhang K, Zu F, et al. Preparation of a poly(ethyleneimine) embedded phenyl stationary phase for mixed-mode liquid chromatography. Anal. Chim. Acta. 2018;1042:165–73.
Bicker W, Lämmerhofer M, Lindner W. Mixed-mode stationary phases as a complementary selectivity concept in liquid chromatography–tandem mass spectrometry-based bioanalytical assays. Anal Bioanal Chem. 2008;390:263–6.
Hinterwirth H, Lämmerhofer M, Preinerstorfer B, Gargano A, Reischl R, Bicker W, et al. Selectivity issues in targeted metabolomics: Separation of phosphorylated carbohydrate isomers by mixed-mode hydrophilic interaction/weak anion exchange chromatography. J. Sep. Sci. 2010;33:3273–82.
Lämmerhofer M, Nogueira R, Lindner W. Multi-modal applicability of a reversed-phase/weak-anion exchange material in reversed-phase, anion-exchange, ion-exclusion, hydrophilic interaction and hydrophobic interaction chromatography modes. Anal Bioanal Chem. 2011;400:2517–30.
Lv Y, Alejandro FM, Fréchet JMJ, Frantisek S. Preparation of porous polymer monoliths featuring enhanced surface coverage with gold nanoparticles. J. Chromatogr. A. 2012;1261:121–8.
Lu J, Ye F, Zhang A, Wei Z, Peng Y, Zhao S. Preparation and characterization of silica monolith modified with bovine serum albumin-gold nanoparticles conjugates and its use as chiral stationary phases for capillary electrochromatography. J. Sep. Sci. 2011;34:2329–36.
Hawes CS, Nolvachai Y, Kulsing C, Knowles GP, Chaffee AL, Marriott PJ, et al. Metal-organic frameworks as stationary phases for mixed-mode separation applications. Chem. Commun. 2014;50:3735–7.
Wu Q, Chen L, Gao J, Dong S, Li H, Di D, et al. Graphene quantum dots-functionalized C18 hydrophobic/hydrophilic stationary phase for high performance liquid chromatography. Talanta. 2019;194:105–13.
Li X, Li B, Liu M, Zhou Y, Zhang L, Qiao X. Core-shell metal-organic frameworks as the mixed-mode stationary phase for hydrophilic interaction/reversed-phase chromatography. ACS Appl. Mater. Interfaces. 2019;11:10320–7.
Liu H, Guo Y, Wang X, Liang X, Liu X, Jiang S. A novel fullerene oxide functionalized silica composite as stationary phase for high performance liquid chromatography. RSC Adv. 2014;4:17541–8.
Li T, Zhuang S, Wang Y, Wang Y, Wang W, Zhang H, et al. Flavonoid profiling of a traditional Chinese medicine formula of Huangqin Tang using high performance liquid chromatography. Acta Pharm. Sin. B. 2016;6:148–57.
Lee K, Kim HY, Lotya M, Coleman JN, Kim GT, Duesberg GS. Electrical characteristics of molybdenum disulfide flakes produced by liquid exfoliation. Adv. Mater. 2011;23:4178–82.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (NO. 21804112), the Project of Department of Education of Sichuan Province (NO. 18ZA0527), the talent introduction program of Southwest Medical University (NO. 090300040040) and the university level fund of Southwest Medical University (NO. 2017-ZRQN-0118).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have are no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 801 kb)
Rights and permissions
About this article
Cite this article
Luo, Q., Ren, X., Wei, S. et al. Preparation and evaluation of a molybdenum disulfide quantum dots embedded C18 mixed-mode chromatographic stationary phase. Anal Bioanal Chem 412, 1365–1374 (2020). https://doi.org/10.1007/s00216-019-02363-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02363-3