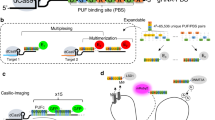
Abstract
The 3D organization of chromatin plays an important role in genome stability and many other pivotal biological programs. Therefore, the establishment of imaging methods, which enable us to study the dynamics of chromatin in living cells, is necessary. Although primary live cell imaging methods were a breakthrough, there is a need to develop more specific labeling techniques. With the discovery of programmable DNA binding proteins, such zinc finger proteins (ZFP), transcription activator-like effectors (TALE), and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9), a major leap forward was made. Here, we review the applications and potential of fluorescent repressor-operator systems, programmable DNA binding proteins with an emphasis on CRISPR-based chromatin imaging in living and fixed cells, and their potential application in plant science.



Similar content being viewed by others
Abbreviations
- BIFC:
-
Bimolecular fluorescence complementation
- CENH3:
-
Centromere specific histone H3
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- Cas9:
-
CRISPR-associated protein 9
- Cys2-His2:
-
Cysteine cysteine-histidine histidine
- crRNA:
-
crisper RNA
- dCas9:
-
dead Cas9
- EdU:
-
5-Ethynyl-2′-deoxyuridine
- FISH:
-
Fluorescence in situ hybridization
- FROS:
-
Fluorescent repressor operator system
- GFP:
-
Green fluorescent protein
- gRNA:
-
guide RNA
- LiveFISH:
-
Live-cell fluorescent in situ hybridization
- Nm:
-
Neisseria meningitides
- TALE:
-
Transcription activator-like effector
- PAM:
-
Protospacer adjacent motif
- PBS:
-
PUF binding site
- PUF:
-
Pumilio/fem-3 mRNA binding factor
- RGEN-ISL:
-
RNA-guided endonuclease-in situ labeling
- RVD:
-
Repeat variable di-residue
- scFv:
-
Single-chain variable fragment antibody
- scFv-GCN4:
-
GCN4 peptide binding single-chain variable fragment antibody
- Sp:
-
Streptococcus pyogenes
- St1:
-
Streptococcus thermophilus
- sgRNA:
-
single guide RNA
- tracr-RNA:
-
Trans-activating RNA
- ZFP:
-
Zinc finger proteins
References
Anton T, Bultmann S, Leonhardt H, Markaki Y (2014) Visualization of specific DNA sequences in living mouse embryonic stem cells with a programmable fluorescent CRISPR/Cas system. Nucleus 5:163–172
Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U (2009) Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326:1509–1512
Boettiger AN, Bintu B, Moffitt JR, Wang S, Beliveau BJ, Fudenberg G, Imakaev M, Mirny LA, Wu CT, Zhuang X (2016) Super-resolution imaging reveals distinct chromatin folding for different epigenetic states. Nature 529:418–422
Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li GW, Park J, Blackburn EH, Weissman JS, Qi LS, Huang B (2013) Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155:1479–1491
Chen H, Choi J, Bailey S (2014) Cut site selection by the two nuclease domains of the Cas9 RNA-guided endonuclease. J Biol Chem 289:13284–13294
Cryderman DE, Morris EJ, Biessmann H, Elgin SCR, Wallrath LL (1999) Silencing at Drosophila telomeres: nuclear organization and chromatin structure play critical roles. EMBO J 18:3724–3735
Deng WL, Shi XH, Tjian R, Lionnet T, Singer RH (2015) CASFISH: CRISPR/Cas9-mediated in situ labeling of genomic loci in fixed cells. Proc Natl Acad Sci U S A 112:11870–11875
Dreissig S, Schiml S, Schindele P, Weiss O, Rutten T, Schubert V, Gladilin E, Mette MF, Puchta H, Houben A (2017) Live-cell CRISPR imaging in plants reveals dynamic telomere movements. Plant J 91:565–573
Duan J, Lu G, Hong Y, Hu Q, Mai X, Guo J, Si X, Wang F, Zhang Y (2018) Live imaging and tracking of genome regions in CRISPR/dCas9 knock-in mice. Genome Biol 19:192
Dvorackova M, Rossignol P, Shaw PJ, Koroleva OA, Doonan JH, Fajkus J (2010) AtTRB1, a telomeric DNA-binding protein from Arabidopsis, is concentrated in the nucleolus and shows highly dynamic association with chromatin. Plant J 61:637–649
Esvelt KM, Mali P, Braff JL, Moosburner M, Yaung SJ, Church GM (2013) Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nat Methods 10:1116
Fu Y, Rocha PP, Luo VM, Raviram R, Deng Y, Mazzoni EO, Skok JA (2016) CRISPR-dCas9 and sgRNA scaffolds enable dual-colour live imaging of satellite sequences and repeat-enriched individual loci. Nat Commun 7:11707
Fujimoto S, Matsunaga S (2017) Visualization of chromatin loci with transiently expressed CRISPR/Cas9 in plants. Cytologia 82:559–562
Fujimoto S, Sugano SS, Kuwata K, Osakabe K, Matsunaga S (2016) Visualization of specific repetitive genomic sequences with fluorescent TALEs in Arabidopsis thaliana. J Exp Bot 67:6101–6110
Gaj T, Gersbach CA, Barbas CF (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31:397–405
Germier T, Sylvain A, Silvia K, David L, Kerstin B (2018) Real-time imaging of specific genomic loci in eukaryotic cells using the ANCHOR DNA labelling system. Methods Enzymol 142:16–23
Gottschling DE, Aparicio OM, Billington BL, Zakian VA (1990) Position effect at Saccharomyces cerevisiae telomeres - reversible repression of Pol-II transcription. Cell 63:751–762
Gu B, Swigut T, Spencley A, Bauer MR, Chung M, Meyer T, Wysocka J (2018) Transcription-coupled changes in nuclear mobility of mammalian cis-regulatory elements. Science 359:1050–1055
Hirakawa T, Katagiri Y, Ando T, Matsunaga S (2015) DNA double-strand breaks alter the spatial arrangement of homologous loci in plant cells. Sci Rep 5:11058
Hong Y, Lu G, Duan J, Liu W, Zhang Y (2018) Comparison and optimization of CRISPR/dCas9/gRNA genome-labeling systems for live cell imaging. Genome Biol 19:39
Ishii T, Schubert V, Khosravi S, Dreissig S, Metje-Sprink J, Sprink T, Fuchs J, Meister A, Houben A (2019) RNA-guided endonuclease - in situ labelling (RGEN-ISL): a fast CRISPR/Cas9-based method to label genomic sequences in various species. New Phytol 222:1652–1661
Jovtchev G, Watanabe K, Pecinka A, Rosin FM, Mette MF, Lam E, Schubert I (2008) Size and number of tandem repeat arrays can determine somatic homologous pairing of transgene loci mediated by epigenetic modifications in Arabidopsis thaliana nuclei. Chromosoma 117:267–276
Jovtchev G, Borisova B, Kuhlmann M, Fuchs J, Watanabe K, Schubert I, Mette MF (2011) Pairing of lacO tandem repeats in Arabidopsis thaliana nuclei requires the presence of hypermethylated, large arrays at two chromosomal positions, but does not depend on H3-lysine-9-dimethylation. Chromosoma 120:609–619
Kato L (2001) Detection of chromosomes tagged with green fluorescent protein in live Arabidopsis thaliana plants. Genome Biol:2
Knight SC, Tjian R, Doudna JA (2018) Genomes in focus: development and applications of CRISPR-Cas9 imaging technologies. Angew Chem 57:4329–4337
Koo DH, Zhao HN, Jiang JM (2016) Chromatin‐associated transcripts of tandemly repetitive DNA sequences revealed by RNA‐FISH. Chromosome Res 24:467–480
Kozubek S, Lukasova E, Amrichova J, Kozubek M, Liskova A, Slotova J (2000) Influence of cell fixation on chromatin topography. Anal Biochem 282:29–38
Lane AB, Strzelecka M, Ettinger A, Grenfell AW, Wittmann T, Heald R (2015) Enzymatically generated CRISPR libraries for genome labeling and screening. Dev Cell 34:373–378
Lassadi I, Kamgoué A, Goiffon I, Tanguy-le-Gac N, KB (2015) Differential chromosome conformations as hallmarks of cellular identity revealed by mathematical polymer modeling. PLoS Comput Biol:1–21
Lindhout BI, Fransz P, Tessadori F, Meckel T, Hooykaas PJJ, Zaal BJ (2007) Live cell imaging of repetitive DNA sequences via GFP-tagged polydactyl zinc finger proteins. Nucleic Acids Res 35:e107
Liu Q, Segal DJ, Ghiara JB, CFB (1997) Design of polydactyl zinc-finger proteins for unique addressing within complex genomes. Proc Natl Acad Sci U S A 94:5525–5530
Ma H, Reyes-Gutierrez P, Pederson T (2013) Visualization of repetitive DNA sequences in human chromosomes with transcription activator-like effectors. Proc Natl Acad Sci U S A 110
Ma HNA, Reyes-Gutierreza P, Wolfec SA, Zhangb S, Pedersona T (2015) Multicolor CRISPR labeling of chromosomal loci in human cells. PNAS 112:3002–3007
Ma H, Tu LC, Naseri A, Huisman M, Zhang S, Grunwald D, Pederson T (2016) Multiplexed labeling of genomic loci with dCas9 and engineered sgRNAs using CRISPRainbow. Nat Biotechnol 34:528–530
Maass PG, Barutcu AR, Shechner DM, Weiner CL, Melé M, Rinn JL (2018) Spatiotemporal allele organization by allele-specific CRISPR live-cell imaging (SNP-CLING). Nat Struct Mol Biol 25:176–184
Mak AN-S, Bradley P, Cernadas RA, Bogdanove AJ, Stoddard BL (2012) The crystal structure of TAL effector PthXo1 bound to its DNA target. Science 335:716–719
Mariamé B, Kappler-Gratias S, Kappler M, Balor S, Gallardo F, Bystricky K (2018) Real-time visualization and quantification of human cytomegalovirus replication in living cells using the ANCHOR DNA labeling technology. J Virol 92:e00571–e00518
Matzke AJM, Huettel B, van der Winden J, Matzke M (2005) Use of two-color fluorescence-tagged transgenes to study interphase chromosomes in living plants. Plant Physiol 139:1586–1596
Miller J, McLachlan AD, Klug A (1985) Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J 4:1609–1614
Miyanari Y, Ziegler-Birling C, Torres-Padilla M-E (2013) Live visualization of chromatin dynamics with fluorescent TALEs. Nat Struct 20:1321–1324
Němečková A, Wäsch C, Schubert V, Ishii T, Hřibová E, Houben A (2019) CRISPR/Cas9-based RGEN-ISL allows the simultaneous and specific visualization of proteins, DNA repeats and sites of. DNA replication, Cytogenetic and Genome Research (in press)
Nimmo ER, Cranston G, Allshire RC (1994) Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J 13:3801–3811
Pecinka A, Kato N, Meister A, Probst AV, Schubert I, Lam E (2005) Tandem repetitive transgenes and fluorescent chromatin tags alter local interphase chromosome arrangement in Arabidopsis thaliana. J Cell Sci 118:3751–3758
Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA (2013) Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183
Qin P, Parlak M, Kuscu C, Bandaria J, Mir M, Szlachta K, Singh R, Darzacq X, Yildiz A, Adli M (2017) Live cell imaging of low- and non-repetitive chromosome loci using CRISPR-Cas9. Nat Commun 8:14725
Robinett CC, Straight AF, Li GW, Willhelm C, Sudlow G, Murray A, Belmont AS (1996) In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J Cell Biol 135:1685–1700
Rosin FM, Watanabe N, Cacas J, Kato N, Arroyo JM, Fang Y, May B, Vaughn M, Simorowski J, Ramu U, McCombie RW, Spector DL, Martienssen RA, Lam E (2008) Genome-wide transposon tagging reveals location-dependent effects on transcription and chromatin organization in Arabidopsis. Plant J
Saad H, GallardoF DM, Tanguy-le-Gac N, Lane D, KB (2014) DNA dynamics during early double-strand break processing revealed by non-intrusive imaging of living cells. PLoS Genet:10
Sanjana NE, Cong L, Zhou Y, Cunniff MM, Feng G, Zhang F (2012) A transcription activator-like effector toolbox for genome engineering. Nat Protoc 7:171–192
Schrumpfova PP, Schorova S, Fajkus J (2016) Telomere- and telomerase-associated proteins and their functions in the plant cell. Front Plant Sci:7
Schubert I, Shaw P (2011) Organization and dynamics of plant interphase chromosomes. Trends Plant Sci 16:273–281
Shao S, Zhang W, Hu H, Xue B, Qin J, Sun C, Sun Y, Wei W, Sun Y (2016) Long-term dual-color tracking of genomic loci by modified sgRNAs of the CRISPR/Cas9 system. Nucleic Acids Res 44:e86
Steinert J, Schiml S, Fauser F, Puchta H (2015) Highly efficient heritable plant genome engineering using Cas9 orthologues from Streptococcus thermophilus and Staphylococcus aureus. Plant J 84:1295–1305
Stella S, Molina R, Yefimenko I, Prieto J, Silva G, Bertonati C, Juillerat A, Duchateau P, Montoya G (2013) Structure of the AvrBs3-DNA complex provides new insights into the initial thymine-recognition mechanism. Acta Crystallogr Sect D 69:1707–1716
Tanenbaum ME, Gilbert LA, Qi LS, Weissman JS, Valle RD (2014) A protein tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159:635
van Tol N, Rolloos M, Hooykaas PJJ, van der Zaal BJ (2019) Two novel strategies to assess meiotic protein in vivo expression in Arabidopsis thaliana. F1000Research 8
Wang S, Su JH, Zhang F, Zhuang X (2016) An RNA-aptamer-based two-color CRISPR labeling system. Sci Rep 6:26857
Wang H, Nakamura M, Abbott TR, Zhao D, Luo A, Yu C, Nguyen CM, Lo A, Daley T, La Russa M, Liu Y, Qi LS (2019a) CRISPR-mediated live imaging of genome editing and transcription. Science 365:1301–1305
Wang S, Hao Y, Zhang L, Wang F, Li J, Wang L, Fan C (2019b) Multiplexed superresolution CRISPR imaging of chromatin in living cells. CCS Chemistry 1:278–285
Wienert B, Wyman SK, Richardson CD, Yeh CD, Akcakaya P, Porritt MJ, Morlock M, Vu JT, Kazane KR, Watry HL, Judge LM, Conklin BR, Maresca M, Corn JE (2019) Unbiased detection of CRISPR off-targets in vivo using DISCOVER-Seq. Science 364:286-289.Wu X., Mao Sh., Ying Y., Ch. K, Chen A.K. (2019) Progress and challenges for live-cell imaging of genomic loci using CRISPR-based platforms. Genomics, Proteomics & Bioinformatics 17: 119–128
Wu X, Mao Sh, Ying Y, ChK, Chen AK (2019) Progress and challenges for live-cell imaging of genomic loci using CRISPR-based platforms. Genomics, Proteomics & Bioinformatics 17:119–128
Xue Y, Murat A (2018) Live-cell imaging of chromatin condensation dynamics by CRISPR. Cell press 4:216–235
Ye H, Rong Z, Lin Y (2017) Live cell imaging of genomic loci using dCas9-SunTag system and a bright fluorescent protein. Protein Cell
Zhang S, Song Z (2017) Aio-Casilio: a robust CRISPR-Cas9-Pumilio system for chromosome labeling. J Mol Histol 48:293–299
Funding
Cytogenetic research in the author’s laboratory has been supported by Deutsche Forschungsgemeinschaft (DFG) grant HO1779/28-1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Beth Sullivan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khosravi, S., Ishii, T., Dreissig, S. et al. Application and prospects of CRISPR/Cas9-based methods to trace defined genomic sequences in living and fixed plant cells. Chromosome Res 28, 7–17 (2020). https://doi.org/10.1007/s10577-019-09622-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-019-09622-0