Abstract
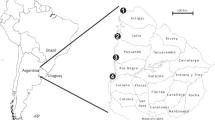
The aim of this study was to detect, quantify, and assess the risk of infection and illness for Group A Rotavirus (RVA) in the watersheds of the Santa Lucia and Uruguay rivers in Uruguay. Monthly sampling was carried out for one year in six sites in the watershed of the Santa Lucía River and four in the Uruguay River. All the collection sites are used for recreational activities. Viral concentration was performed with the adsorption–elution method, and detection and quantification of RVA was carried out by TaqMan quantitative PCR (qPCR). Quantitative microbial risk assessment was applied to estimate the daily and annual risk of RVA infection, as well as the daily risk of illness considering direct exposure through recreational activity. RVA was detected in 42% (20/48) of the analyzed samples in the Uruguay River and 40% (29/72) in the Santa Lucía River. The virus was present in all the analyzed points in both watersheds. A pattern of seasonality, characterized by a higher detection frequency of the virus during coldest month of the year, was observed in both basins. The mean concentration for RVA was 1.3 × 105 genomic copies/L. The microbiological risk assessment shows that Santa Lucía watershed presented the highest daily risk of infection (6.41E–01) and illness (3.20E–01) estimated for the point downstream of Florida City; meanwhile for Uruguay River, the highest probabilities of infection (6.82E–01) and illness (3.41E–01) were estimated for the collection site for drinking water intake in Salto city. These results suggest that RVA contamination of these important rivers negatively impact on their microbiological quality since they are used for recreation and drinking water intake, demonstrating that the disposal of waste from cities located in their riverside confers a constant threat of infection for the general population, especially for children.



Similar content being viewed by others
References
Ansari, S. A., Springthorpe, V. S., & Sattar, S. A. (1991). Survival and vehicular spread of human rotaviruses: Possible relation to seasonality of outbreaks. Reviews of Infectious Diseases,13(3), 448–461.
Assis, A. S., Cruz, L. T., Ferreira, A. S., Bessa, M. E., de Oliveira Pinto, M. A., Vieira, C. B., et al. (2015). Relationship between viral detection and turbidity in a watershed contaminated with group A rotavirus. Environmental Science and Pollution Research,22(9), 6886–6897.
Aubriot, L., Delbene, L., Haakonsson, S., Somma, A., Hirsch, F., Bonilla, S. (2017). Evolución de la eutrofización en el Río Santa Lucía: influencia de la intensificación productiva y perspectivas. Innotec, (14).
Barril, P. A., Fumian, T. M., Prez, V. E., Gil, P. I., Martínez, L. C., Giordano, M. O., et al. (2015). Rotavirus seasonality in urban sewage from Argentina: Effect of meteorological variables on the viral load and the genetic diversity. Environmental Research,138, 409–415. https://doi.org/10.1016/j.envres.2015.03.004.
Bortagaray, V., Lizasoain, A., Piccini, C., Gillman, L., Berois, M., Pou, S., et al. (2019). Microbial source tracking analysis using viral indicators in Santa Lucía and Uruguay rivers, Uruguay. Food and Environmental Virology. https://doi.org/10.1007/s12560-019-09384-2
Braeye, T., Schrijver, D. E. K., Wollants, E., van Ranst, M., & Verhaegen, J. (2015). A large community outbreak of gastroenteritis associated with consumption of drinking water contaminated by river water, Belgium, 2010. Epidemiology and Infection,143(4), 711–719.
C.A.R.U. Comisión Administradora del Río Uruguay. (2018). El Río Uruguay en cifras. Retrieved January 15, 2018 from https://www.caru.org.uy/web/acerca-de/prueba-pagina
Carlton, E. J., Woster, A. P., DeWitt, P., Goldstein, R. S., & Levy, K. (2016). A systematic review and meta-analysis of ambient temperature and diarrhoeal diseases. International Journal of Epidemiology,45, 117–130.
Chigor, V. N., Sibanda, T., & Okoh, A. I. (2014). Assessment of the risks for human health of adenoviruses, hepatitis A virus, rotaviruses and enteroviruses in the Buffalo River and three source water dams in the Eastern Cape. Food and Environmental Virology,6, 87–98.
Cook, S. M., Glass, R. I., LeBaron, C. W., et al. (1990). Global seasonality of rotavirus infections. Bulletin of the World Health Organization,68(2), 171–177.
De La Cruz Hernández, S. I., Anaya Molina, Y., Gómez Santiago, F., Terán Vega, H. L., Monroy Leyva, E., Méndez Pérez, H., et al. (2018). Real-time RT-PCR, a necessary tool to support the diagnosis and surveillance of rotavirus in Mexico. Diagnostic Microbiology and Infectious Disease,90(4), 272–276. https://doi.org/10.1016/j.diagmicrobio.2017.12.005.
Divizia, M., Gabrieli, R., Donia, D., Macaluso, A., Bosch, A., Guix, S., et al. (2004). Waterborne gastroenteritis outbreak in Albania. Water Science and Technology,50(1), 57–61.
Elmahdy, M. E., Fongaro, G., Magri, M. E., Petruccio, M. M., & Barardi, C. R. (2016). Spatial distribution of enteric viruses and somatic coliphages in a Lagoon used as drinking water source and recreation in Southern Brazil. International Journal of Hygiene and Environmental Health, 219(7 Pt A), 617–625.
Espinosa, A. C., Mazari-Hiriart, M., Espinosa, R., Maruri-Avidal, L., Méndez, E., & Arias, C. F. (2008). Infectivity and genome persistence of rotavirus and astrovirus in groundwater and surface water. Water Research,42(10–11), 2618–2628. https://doi.org/10.1016/j.watres.2008.01.018.
Estes, M. K., & Greenberg, H. B. (2013). Rotaviruses. In D. M., Knipe, P. M., Howley, J. I., Cohen, D. E., Griffin, R. A., Lamb, M. A., Martin, et al. (Eds.), Fields virology (6th ed.). Philadelphia: Lippincott Williams and Wilkins.
Fewtrell, L., & Kay, D. (2015). Recreational water and infection: A review of recent findings. Current Environmental Health Reports,2, 85–94.
Fong, T. T., & Lipp, E. K. (2005). Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiology and Molecular Biology Reviews,69(2), 357–371.
Fongaro, G., Nascimento, M. A., Viancelli, A., Tonetta, D., Petrucio, M. M., & Barardi, C. R. (2012). Surveillance of human viral contamination and physicochemical profiles in a surface water lagoon. Water Science and Technology,66(12), 2682–2687. https://doi.org/10.2166/wst.2012.504.
Fongaro, G., Padilha, J., Schissi, C. D., Nascimento, M. A., Bampi, G. B., Viancelli, A., et al. (2015). Human and animal enteric virus in groundwater from deep wells, and recreational and network water. Environmental Science and Pollution Research,22, 20060–20066.
Fumian, T. M., Leite, J. P. P., Rose, T. L., Prado, T., & Miagostovich, M. P. (2011). One year environmental surveillance of rotavirus species A (RVA) genotypes in circulation after the introduction of the Rotarix vaccine in Rio de Janeiro, Brazil. Water Research,45, 5755–5763.
Gerba, C. P. (2019). Risk assessment. In M. L. Brusseau, I. L. Pepper, & C. Gerba (Eds.), Environmental and pollution science (3rd ed., pp. 541–563). Amsterdam: Academic Press.
Gerba, C. P., Rose, J. B., Haas, C. N., et al. (1996). Waterborne rotavirus: A risk assessment. Water Research,12, 2929–2940.
Haas, C. N., Rose, J. B., Gerba, C., et al. (1993). Risk assessment of virus in drinking water. Risk Analysis,13(5), 545–552.
Haas, C. N., Rose, J. B., & Gerba, C. P. (1999). Quantitative microbial risk assessment. New York: Wiley.
Haramoto, E., Katayama, H., Utagawa, E., & Ohgaki, S. (2009). Recovery of human norovirus from water by virus concentration methods. Journal of Virological Methods,160(1–2), 206–209. https://doi.org/10.1016/j.jviromet.2009.05.002.
Katayama, H., Shimasaki, A., & Ohgaki, S. (2002). Development of a virus concentration method and its application to detection of enterovirus and norwalk virus from coastal seawater. Applied and Environmental Microbiology,68(3), 1033–1039.
Kiulia, N. M., Hofstra, N., Vermeulen, L. C., Obara, M. A., Medema, G., & Rose, J. B. (2015). Global occurrence and emission of rotaviruses to surface waters. Pathogens,4(2), 229–255. https://doi.org/10.3390/pathogens4020229.
Koroglu, M., Yakupogullari, Y., Otlu, B., Ozturk, S., Ozden, M., Ozer, A., et al. (2011). A waterborne outbreak of epidemic diarrhea due to group A rotavirus in Malatya, Turkey. New Microbiologica,34(1), 17–24.
Kotloff, K. L., Nataro, J. P., Blackwelder, W. C., Nasrin, D., Farag, T. H., Panchalingam, S., et al. (2013). Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet,382(9888), 209–222.
Kraay, A. N. M., Brouwer, A. F., Lin, N., Collender, P. A., Remais, J. V., & Eisenberg, J. N. S. (2018). Modeling environmentally mediated rotavirus transmission: The role of temperature and hydrologic factors. Proceedings of the National Academy of Sciences of the United States of America,115(12), E2782–E2790.
Levy, K., Hubbard, A. E., & Eisenberg, J. N. (2009). Seasonality of rotavirus disease in the tropics: A systematic review and meta-analysis. International Journal of Epidemiology,38(6), 1487–1496. https://doi.org/10.1093/ije/dyn260.
Martinelli D, Prato R, Chironna M, Sallustio A, Caputi G, Conversano M et al (2007) Large outbreak of viral gastroenteritis caused by contaminated drinking water in Apulia, Italy, May–October 2006. Euro Surveillance, 12(4):E070419.1.
Masachessi, G., Pisano, M. B., Prez, V. E., Martínez, L. C., Michelena, J. F., Martínez-Wassaf, M., et al. (2018). Enteric viruses in surface waters from Argentina: Molecular and viable-virus detection. Applied and Environmental Microbiology. https://doi.org/10.1128/AEM.02327-17
Mellou, K., Katsioulis, A., Potamiti-Komi, M., Pournaras, S., Kyritsi, M., Katsiaflaka, A., et al. (2014). A large waterborne gastroenteritis outbreak in central Greece, March 2012: Challenges for the investigation and management. Epidemiology and Infection,142(1), 40–50.
Miagostovich, M. P., Ferreira, F. F., Guimarães, F. R., Fumian, T. M., Diniz-Mendes, L., Luz, S. L., et al. (2008). Molecular detection and characterization of gastroenteritis viruses occurring naturally in the stream waters of Manaus, central Amazonia, Brazil. Applied and Environmental Microbiology,74(2), 375–382.
Ming, H. X., Zhu, L., Feng, J. F., Yang, G., & Fan, J. F. (2014). Risk assessment of rotavirus infection in surface seawater from Bohai Bay, China. Human and Ecological Risk Assessment,20, 929–940.
Miura, T., Gima, A., & Akiba, M. (2019). Detection of norovirus and rotavirus present in suspended and dissolved forms in drinking water sources. Food and Environmental Virology,11(1), 9–19.
MSP. (2018). Vacunas. Retrieved November 24, 2018 from https://www.msp.gub.uy/noticia/vacunas
Pang, X., Qiu, Y., Gao, T., Zurawell, R., Neumann, N. F., Craik, S., et al. (2019). Prevalence, levels and seasonal variations of human enteric viruses in six major rivers in Alberta, Canada. Water Research,153, 349–356.
Platts-Mills, J. A., Babji, S., Bodhidatta, L., Gratz, J., Haque, R., Havt, A., et al. (2015). MAL-ED Network Investigators. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). The Lancet Global Health, 3(9), e564–e575.
Prez, V. E., Gil, P. I., Temprana, C. F., et al. (2015). Quantification of human infection risk caused by rotavirus in surface waters from Córdoba, Argentina. Science of the Total Environment,538, 220–229.
Reynolds, K. A. (2004). Integrated cell culture/PCR for detection of enteric viruses in environmental samples. In J. F. T. Spencer & A. R. Spencer (Eds.), Public health microbiology (pp. 69–78). Totowa: Humana Press.
Rigotto, C., Victoria, M., Moresco, V., et al. (2010). Assessment of adenovirus, hepatitis A virus and rotavirus presence in environmental samples in Florianopolis, South Brazil. Journal of Applied Microbiology,109, 1979–1987.
Rutjes, S. A., Lodder, W. J., Docters van Leeuwen, A., & Roda Husman, A. M. (2009). Detection of infectious rotavirus in naturally contaminated source waters for drinking water production. Journal of Applied Microbiology,107, 97–105.
Sinclair, R. G., Jones, E. L., & Gerba, C. P. (2009). Viruses in recreational water-borne disease outbreaks: A review. Journal of Applied Microbiology,107(6), 1769–1780.
Staggemeier, R., Heck, T. M. S., Demoliner, M., Ritzel, R. G. F., Röhnelt, N. M. S., Girardi, V., et al. (2017). Venker CA, Spilki FR. Enteric viruses and adenovirus diversity in waters from 2016 Olympic venues. Science of the Total Environment,586, 304–312.
Tort, L. F., Victoria, M., Lizasoain, A., García, M., Berois, M., Cristina, J., et al. (2015a). Detection of common, emerging and uncommon VP4, and VP7 human Group A Rotavirus genotypes from urban sewage samples in Uruguay. Food and Environmental Virology,7(4), 342–353. https://doi.org/10.1007/s12560-015-9213-5.
Tort, L. F., Victoria, M., Lizasoain, A. A., Castells, M., Maya, L., Gómez, M. M., et al. (2015b). Molecular epidemiology of group A rotavirus among children admitted to hospital in Salto, Uruguay, 2011–2012: First detection of the emerging genotype G12. Journal of Medical Virology,87(5), 754–763.
Victoria, M., Fumian, T. M., Rocha, M. S., Dalmao, F., Leite, J. P., Girones, R., et al. (2014). Gastroenteric virus dissemination and influence of rainfall events in urban beaches in Brazil. Journal of Applied Microbiology,117(4), 1210–1218.
Vieira, C. B. (2015). Rastreamento microbiológico de fontes de contaminação humana e animal por marcadores virais e avaliação de risco de infecções por vírus gastroentéricos na bacia do Rio Negro, Manaus, Amazonas. PhD Thesis.
Vieira, C. B., deAbreu, C. A., de Jesus, M. S., Luz, S. L., Wyn-Jones, P., Kay, D., et al. (2016). Viruses surveillance under different season scenarios of the Negro River Basin, Amazonia, Brazil. Food and Environmental Virology,8, 57–69.
Ward, R. L., Bernstein, D. I., Young, E. C., et al. (1986). Human rotavirus studies in volunteers: Determination of infectious dose and serological response to infection. The Journal of Infectious Diseases,154(5), 871–880.
Zeng, S. Q., Halkosalo, A., Salminen, M., et al. (2008). One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. Journal of Virological Methods,153(2), 238–240.
Acknowledgements
We want to thank the “Comisión Sectorial de Investigación Científica”, Project I+D 2014 (ID 287), Universidad de la República, Uruguay, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bortagaray, V., Girardi, V., Pou, S. et al. Detection, Quantification, and Microbial Risk Assessment of Group A Rotavirus in Rivers from Uruguay. Food Environ Virol 12, 89–98 (2020). https://doi.org/10.1007/s12560-019-09416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-019-09416-x