Abstract
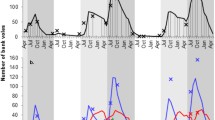
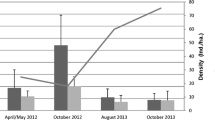
The cricetid rodent Oligoryzomys longicaudatus is the species host of Andes virus (ANDV) which causes hantavirus pulmonary syndrome in southern Argentina and Chile. Population density, behavioral interactions, and spacing patterns are factors that affect viral transmission among wild rodents. We predict that the highest prevalence of hantavirus antibody positive would be found among wounded, reproductive males and that, at high population densities, wounded, reproductive males would be dispersers rather than resident individuals. The study was conducted seasonally from October (spring) 2011 to October (spring) 2013 in a shrubland habitat of Cholila, Argentina. During each trapping session, we classified captured O. longicaudatus as resident or disperser individuals, estimated population density, and recorded wounds as an indicator of aggression among individuals. We obtained blood samples from each individual for serological testing. We used generalized linear models to test the statistical significance of association between antibody prevalence, and sex, resident/dispersal status, wounds and trapping session. The highest proportion of seropositive O. longicaudatus individuals was among wounded reproductive males during periods of the greatest population density, and the characteristics of seroconverted individuals support that transmission is horizontal through male intrasexual competition. A positive association between dispersing individuals and hantavirus antibody was detected at high population density. Our study design allowed us to obtain data on a large number of individuals that are seroconverted, enabling a better understanding of the ecology and epidemiology of the ANDV host system.


Similar content being viewed by others
References
Abbot KD, Ksiazek TG, Mills J (1999) Long term hantavirus persistence in rodent populations in Central Arizona. Emerging Infectious Diseases 5:8–18
Agrell J, Erlinge S, Nelson S, Sandell M (1996) Shifting spacing behaviour of male field voles (Microtus agrestis) over the reproductive season. Annales Zoologici Fennici 33:243–248
Andreo V, Glass G, Shields T, Provensal C, Polop J (2011) Modeling potential distribution of Oligoryzomys longicaudatus, the Andes virus reservoir, in Argentina. EcoHealth 8 (3): 332–348. https://doi.org/10.1007/s10393-011-0719-5
Andreo V, Provensal C, Levis S, Pini N, Enría D, Polop J (2012) Summer–autumn distribution and abundance of the hantavirus host, Oligoryzomys longicaudatus, in northwestern Chubut, Argentina. Journal of Mammalogy 93:1559–1568
Andreo V, Neteler M, Rocchini D, Provensal C, Levis S, Porcasi X, Rizzoli A, Lanfri M, Scavuzzo M, Pini N, Enria D, Polop J. (2014) Estimating Hantavirus Risk in Southern Argentina: A GIS-Based Approach Combining Human Cases and Host Distribution. Viruses 6:201–222; https://doi.org/10.3390/v6010201
Austrich A, Steinmann AR, Bonatto F, Gomez D (2014) Efecto de adultos en el establecimiento de juveniles de Calomys musculinus. Mastozoología Neotropical 21:101–107
Bagamian KH, Towner JS, Kuenzi AJ, Douglass RJ, Rollin PE, Waller LA, Mills JN (2012) Transmission ecology of Sin Nombre hantavirus in naturally infected North American deer mouse. Populations in outdoor enclosures. PLOS ONE. www.plosone.org
Barton K (2018) R package multi-model inference, Version 1.42. Encoding UTF-8. https://CRAN.R-project.org/package=MuMIn
Biggs JR, Bennett KD, Mullen MA, et al. (2000) Relationship of ecological variables to Sin Nombre Virus antibody seroprevalence in populations of deer mice. Journal of Mammalogy 81:676–682
Blanchard R, Blanchard C (1977) Aggressive Behavior in Rat. Behavioral Biology 21:197–224
Bonatto F, Coda J, Gomez D, Priotto J, Steinmann AR (2013) Inter-male aggression with regard to polygynous mating system in Pampean grassland mouse, Akodon azarae (Cricetidae: Sigmodontinae). Journal of Ethology 31:223–231
Bonatto F, Steinmann AR, Gomez D, Priotto J (2015) Do polygynous males of Akodon azarae (Rodentia: Sigmodontinae) vary their mating tactics at low availability of females? Mammalia 79:159–168
Bondrup-Nielsen S (1985) An evaluation of the effects of space use and habitat patterns on dispersal in small mammals. Annales Zoologici Fennici 22:373–383
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information–theoretic approach. Springer, Berlin
Calderón G, Pini N, Bolpe J, Levis S, Mills J, Segura E, Guthmann N, Cantón G, Bécker J, Fonollat A, Ripoll C, Bortman M, Benedetti R, Sabattini M, Enria D (1999) Hantavirus reservoir hosts associated with peridomestic habitats in Argentina. Emerging Infectious Diseases 5:792–797
Calisher CH, Sweeney WP, Mills JN, Beaty BJ (1999) Natural history of Sin Nombre virus in western Colorado. Emerging Infectious Diseases 5:126–134
Cantoni G, Padula P, Calderón G, Mills J, Herrero E, Sandoval P, et al. (2001) Seasonal variation in prevalence of antibody to hantaviruses in rodents from southern Argentina. Tropical Medicine and International Health 6:811–846
Clobert J, Baguette M, Benton T, Bullock J, Ducatez S (2012) Dispersal ecology and evolution. Oxford University Press, Oxford.
Clutton-Brock TH (1989) Mammalian mating systems. Proceedings of the Royal Society of London Biological Sciences 236:339–372
Dewsbury DA (1988) Kinship, Familiarity, Agression, and Dominance in Deer Mice (Peromyscus maniculatus) in Seminatural Enclosures. Journal of Comparative Psychology 102:124–128
Douglas RJ, Wilson T, Semmens WJ, Santo SN, Bond CW, Van Horn RC, Mills JN (2001) Longitudinal studies of Sin Nombre virus in deermouse-dominated ecosystems of Montana. American Journal of Tropical Medicine and Hygiene 65:33–392
Douglass RJ, Kuenzi AJ, Williams CY, Douglass SJ (2003) Removing deer mice from buildings: potential effects on risk of human exposure to Sin Nombre virus. Emerging Infectious Diseases 9:390–392
Douglass RJ, Calisher CH, Wagoner KD, Mills JN (2007) Sin nombre virus infection of deer mice in montana: characteristics of newly infected mice, incidence, and temporal pattern of infection. Journal of Wildlife Diseases 43:12–22
Emlen ST, Oring LW (1977) Ecology, sexual selection and the evolution of mating systems. Science 197:215–223
Enría D, Levis S (2004) Zoonosis virales emergentes: las infecciones por hantavirus. Revue Scientifique et Technique (International Office of Epizootics) 23:595–611
Enría DA, Pinheiro F (2000) Rodent-borne emerging viral zoonosis: hemorrhagic fevers and hantavirus infections in South America. In: Infectious disease clinics of North America. Emerging and reemerging diseases in Latin America, Gotuzzo E, Istúriz R (editors), Philadelphia, PA: WB Saunders, Vol. 14(1), pp 167–184
Farias V, Fuller TK, Cervantes FA, Lorenzo C (2006) Home range and social behavior of the endangered Tehuantepec jackrabbit (Lepus flavigularis) in Oaxaca, México. Journal of Mammalogy 87:748–756
Glass GE (1997) Hantaviruses. Current Opinion in Infectious Diseases 10:362–366
Glass GE, Childs JE, Korch, GW, LeDuc JW (1988) Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvergicus). Epidemiology and Infection 101:459–72
Glass GE, Shields T, Cai B, Yates TL, et al. (2007) Persistently highest risk areas for hantavirus pulmonary syndrome: potential sites for refugia. Ecological Applications 17:129–139
Guzman C, Mattar S, Levis S, Pini N, Figueiredo T, Mills J, Salazar-Bravo J (2013) Prevalence of antibody to hantaviruses in humans and rodents in the Caribbean region of Colombia determined using Araraquara and Maciel virus antigens. Memórias do Instituto Oswaldo Cruz 108:167–171.
Hinson ER, Shon SM, Zink MC, Glass GE, Klein SL (2004) Wounding: the primary mode of Seoul virus transmission among male Norway rats. American Journal of Tropical Medicine and Hygiene 70:310–317
Juan EE, Provensal MC, Steinmann AR (2018) Space Use and Social Mating System of the Hantavirus Host, Oligoryzomys longicaudatus. EcoHealth 15:96–108
Kallio ER, Klingström J, Gustafsson E, Manni T, Vaheri A, Henttonen H, Lundkvist A (2006) Prolonged survival of Puumala hantavirus outside the host: evidence for indirect transmission via the environment. Journal of General Virology 87:2127–2134
Korpela K, Sundell J., Ylönen H (2011) Does personality in small rodents vary depending on population density? Oecologia 165:67–77
Kuenzi AJ, Morrison ML, Swann DE, Hardy PC, Downard GT (1999) A longitudinal study of Sin Nombre Virus prevalence in rodents, Southeastern Arizona. Emerging Infectious Diseases 5:113–117
Lambin X, Aars J, Piertney SB (2001) Dispersal, intraspecific competition, kin competition and kin facilitation: A review of the empirical evidence. In: Dispersal, Clobert J, Danchin E, Dhondt A, Nichols JD (editors), Oxford: Oxford University Press, pp 110–22
Lázaro ME, Cantoni GE, Calanni LM, Resa AJ, Herrero ER, Iacono MA, Enría DA, González Cappa SM (2007) Clusters of hantavirus infection, southern Argentina. Emerging Infectious Diseases 13:104–110
Levis S, Rowe JE, Morzunov S, Enría DA, St. Jeor S (1997) New hantaviruses causing hantavirus pulmonary syndrome in central Argentina. The Lancet 349: 998–999
Levis S, Morzunov S, Rowe J, Enria D, Pini N, Calderón G, Sabattini M, St Jeor S (1998) Genetic diversity and epidemiology of hantaviruses in Argentina. Journal of Infectious Diseases 177:529–538
Lidicker WZ (1975) The role of dispersal in the demography of small mammals. In: Golley FB, Petrusewicz K, Ryszkowski L (eds) Small Mammals: Their Productivity and Population Dynamics. Cambridge University Press, London, pp 103–128
Lidicker WZ, Stenseth NC (1992) To disperse or not to disperse: who does it and why? In: Animal dispersal: small mammals as a model, Stenseth NC, Lidicker WZ (editors), London: Chapman and Hall, pp 21–36
Lonner BN, Douglass RJ, Kuenzi AJ, Hughes K (2008) Seroprevalence against Sin Nombre Virus in resident and dispersing deer mice. Vector Borne and Zoonotic Diseases 8:433– 441
Loughran MFE (2007) Social organization of the male field vole (Microtus agrestis): a case of transient territoriality?. Annals of Zoologici Fennici 44:97–106
Maes P, Adkins S, Alkhovsky SV, Avšič‑Županc T, Ballinger MJ (2019) Taxonomy of the order Bunyavirales: second update 2018. Archives of Virology 164:927–941
Mills JN, Childs JE, Enria DA, Bowen MD, Peters CJ, Ksiazek TG, Jahrling PB (1994) Oliveros virus: seroprevalence of a new arenavirus in rodents and humans in central Argentina. American Journal of Tropical Medicine and Hygiene 51:96–97
Mills JN, Yates TL, Childs JE, Parmenter RR, Ksiazek TG, et al. (1995) Guidelines for working with rodents potential infected with hantavirus. Journal of Mammalogy 76:716–722
Mills JN, Ksiazek TG, Ellis BA, Rollin PE, Nichol ST, Yates TL, Gannon WL, Levy CE, Engelthaler DM, Davis T, Tanda DT, Frampton JW, Nichols CR, Peters CJ, Childs JE. et al. (1997) Patterns of association with host and habitat: antibody reactive with Sin Nombre virus in small mammals in the major biotic communities of the Southwestern United States. American Journal of Tropical Medicine and Hygiene 56:273–84
Mills JN, Ksiazek TG, Peters CJ, Childs JE (1999) Long-term studies of hantavirus reservoir populations in the southwestern United States: a synthesis. Emerging Infectious Diseases 5:135–142
Mills JN, Schmidt K, Ellis BA, Calderón G, Enría DA, Ksiazek TG (2007) A longitudinal study of hantavirus infection in three sympatric reservoir species in agroecosystems on the Argentine Pampa. Vector-Borne and Zoonotic Diseases 7:229–240
Mills JN, Amman BR, Glass GE (2010) Ecology of Hantaviruses and their hosts in North America. Vector-Borne and Zoonotic Diseases 10, No. 6, published Online-2010. https://doi.org/10.1098/vbz.2009.0018
Murúa R, Briones M (2005) Abundance of the sigmodont mouse Oligoryzomys longicaudatus and patterns of tree seeding in Chilean temperate forest. Mammalian Biology 70:321–326
Murúa R, Navarrete M, Cadiz R, Figueroa R, Padula, Zaror L, Mancilla R, González L, Muñoz-Pedreros A (2003) Hantavirus pulmonary syndrome: Current situation among rodent reservoirs and human population in the Xth Region, Chile. Revista Médica de Chile 131:169–176
Padula P, Colavecchia SB, Martínez VP, Gonzalez Della Valle MO, Edelstein A, et al. (2000) Genetic diversity, distribution, and serological features of hantavirus infection in five countries in South America. Journal of Clinical Microbiology 38:3029–3035
Padula P, Figueroa R, Navarrete M, Pizarro E, Cadiz R, Bellomo C, Jofre C, Zaror L, Rodriguez E, Murúa R (2004) Transmission study of andes hantavirus infection in wild sigmodontine rodents. Journal of Virology 90:11972–11979
Pearson OP (2002) A perplexing outbreak of mice in Patagonia, Argentina. Studies on Neotropical Fauna and Environment 37:187–200
Piudo L, Monteverde M, Gonzalez Capria S, Padula P, Carmanchahi P (2005) Distribution and abundance of sigmodontine rodents in relation to hantavirus in Neuquén, Argentina. Journal of Vector Ecology 30:119–125
Piudo L, Monteverde MJ, Walker RS, Douglass RJ (2011) Rodent community structure and Andes virus infection in sylvan and peridomestic habitats in Northwestern Patagonia, Argentina. Vector-borne and Zoonotic Diseases 11:315–324
Piudo L, Monteverde MJ, Walker RS, Douglass RJ (2012) Características de Oligoryzomys longicaudatus asociadas a la presencia del virus Andes (Hantavirus). Revista Chilena de Infectología 29:200–206
Polop F, Provensal C, Pini N, Levis S, Priotto JW, Enría D, Calderón GE, Costa F, Polop JJ (2010) Temporal and spatial host abundance and prevalence ofAndes Hantavirus in southern Argentina. EcoHealth 7:176–184
Polop F, Juan J, Polop J, Provensal MC (2014a) Spatial and temporal variation of terrestrial rodent assemblages in Cholila, Chubut Province, Argentina. Studies on Neotropical Fauna and Environment 49:151–157
Polop F, Sepúlveda L, Pelliza Sbriller A, Polop J, Provensal MC (2014b) Food habits of Oligoryzomys longicaudatus (Rodentia) in a steppe-forest transitional area of Argentinean Patagonia. Ecología Austral 24:304–310
Polop F, Levis S, Pini N, Enría D, Polop J, Provensal MC (2018) Factors associated with hantavirus infection in a wild host rodent from Cholila, Chubut Province, Argentina. Mammalian Biology 88:107–113
Priotto JW, Steinmann AR, Provensal C, Polop J (2004) Juvenile dispersal in Calomys venustus (Muridae: Sigmodontinae). Acta Oecologica 25:205–210
R Development Core Team (2010) R: a language and environment for statistical computing, Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/. Accessed March 2011
Sage RD, Pearson OP, Sanguinetti J, Pearson AK (2007) Ratada 2001: a rodent outbreak following the flowering of bamboo (Chusquea culeou) in southwestern Argentina. In: The Quintessential Naturalist: Honoring the Life and Legacy of Oliver P. Pearson, Kelt DA, Lessa EP, Salazar-Bravo J, Patton JL (editors), California: University of California Publications in Zoology, pp 177–224
Schmaljohn C, Hjelle B (1997) Hantaviruses: a global disease problem. Emerging Infectious Diseases 3:95–104
Schooley RL, Branch LC (2006) Space use by round-tailed muskrats in isolated wetlands. Journal of Mammalogy 87:495–500
Steinmann AR, Priotto J (2011) Inter-male aggression in relation to female availability and residence status in corn mice, Calomys musculinus. Acta Theriologica 56: 81–89
Steinmann AR, Priotto JW, Sommaro L, Polop J (2006a) Spacing behaviour of juveniles corn mice Calomys musculinus at the beginning of the breeding period, in absence of adult males. Acta Oecologica 29:305–310
Steinmann AR, Priotto JW, Sommaro L, Polop J (2006b) The influence of adult female absence on the spacing behavior of juvenile corn mice, Calomys musculinus: a removal experiment. Annales Zoologici Fennici 43:366–372
Steinmann AR, Priotto J, Polop J (2009) Territorial behaviour in corn mice, Calomys musculinus (Muridae: Sigmodontinae), with regard to mating system. Journal of Ethology 27: 51–58
Stenseth N, Lidicker W Jr. (1992) Animal Dispersal. Small mammals as a model. Chapman and Hall.
Stickel L (1954) A comparison of certain methods of measuring ranges of small mammals. Journal of Mammalogy 35:1–5
Stickel L (1968a) Dispersal of the old field mouse. In: The Biology of Peromyscus, King JA (editor), Spec. Publ. No. 2, American Society of Mammalogists 373–411
Stickel L (1968b) Home range and travels. In: The Biology of Peromyscus, King JA (editor), Spec. Publ. No. 2, American Society of Mammalogists 412–456
Torres-Perez FJ, Navarrete-Droguett J, Aldunate R, Yates TL, Mertz GJ, Vial PA, et al. (2004) Peridomestic small mammals associated with confirmed cases of human hantavirus disease in southcentral Chile. American Journal of Tropical Medicine and Hygiene 70:305–309
Van Zegeren K (1980) Variation in aggressiveness and the regulation of numbers in house mouse populations. Netherlands Journal of Zoology 30:635–770
Waterman J (2007) Male Mating Strategies in rodents. In: Rodent Societies. An Ecological, Evolutionary Perspective, Wolff JO, Sherman PW (editors), University of Chicago Press, pp 27–41
Williams J, Bryan R, Mills J, Palma E, Vera I, Peters C, Zaki S, Khan A, Ksiazek T (1997) An outbreak of hantavirus pulmonary syndrome in western Paraguay. American Journal of Tropical Medicine and Hygiene 57:274–282
Wolff JO (1999) Behavioral model systems. In: Barret GW, Peles JD (eds) Landscape Ecology of Small Mammals. Springer, Berlin, pp 11–40
Wolff JO (2003) Density-dependence and the socioecology of space use in rodents. In: Rats, mice and people: rodent biology and management, Singleton GR, Hinds LA, Krebs C, Spratt D (editors), Australian Centre for International Agricultural Research, Canberra, pp 124–130
Wolff JO (2007) Social biology of rodents. Integrative Zoology 2:193–204
Wolff JO, Summerlin CT (1993) Agonistic behavior in organized and disorganized cotton rat populations. Science 160:98–99
Young JC, Mills JN, Enria DA, Dolan NE, Khan AS, Ksiazek TG (1998) Newworld hantaviruses. British Medical Bulletin 54:659–673
Zar JH (1996) Biostatistical analysis. Third ed. Prentice-Hall, Upper Saddle River, New Jersey, USA.
Acknowledgements
This study was supported by Fondo para la Investigación Científica y Tecnológica (FONCYT), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Fundación Mundo Sano. We thank Simon E. Gutiérrez-Brida for assistance with revision of the English version and the associate editor and the anonymous reviewers for helpful comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Juan, E., Levis, S., Pini, N. et al. Mechanisms of Hantavirus Transmission in Oligoryzomys longicaudatus. EcoHealth 16, 671–681 (2019). https://doi.org/10.1007/s10393-019-01454-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-019-01454-y