Abstract
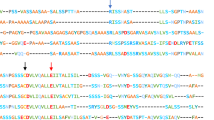
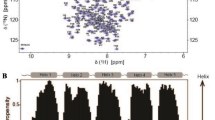
Web spiders use specialized glands to produce silk proteins, so-called spidroins, which assemble into extraordinarily tough silk fibers through tightly regulated phase and structural transitions. A crucial step in the polymerization of spidroins is the pH-triggered assembly of their N-terminal domains (NTDs) into tight dimers. Major ampullate spidroin NTDs contain an unusually high content of the amino acid methionine. We previously showed that the simultaneous mutation of the six hydrophobic core methionine residues to leucine in the NTD of the major ampullate spidroin 1 from Euprosthenops australis, a nursery web spider, yields a protein (L6-NTD) retaining a three-dimensional fold identical to the wildtype (WT) domain, yet with a significantly increased stability. Further, the dynamics of the L6-NTD are significantly reduced and the ability to dimerize is severely impaired compared to the WT domain. These properties lead to significant changes in the NMR spectra between WT and L6-NTD so that the previously available WT-NTD assignments cannot be transferred to the mutant protein. Here, we thus report the de novo NMR backbone and side chain assignments of the major ampullate spidroin 1 L6-NTD variant from E. australis as a prerequisite for obtaining further insights into protein structure and dynamics.


Similar content being viewed by others
References
Askarieh G, Hedhammar M, Nordling K, Saenz A, Casals C, Rising A, Johansson J, Knight SD (2010) Self-assembly of spider silk proteins is controlled by a pH-sensitive relay. Nature 465:236–238. https://doi.org/10.1038/nature08962
Hagn F, Eisoldt L, Hardy JG, Vendrely C, Coles M, Scheibel T, Kessler H (2010) A conserved spider silk domain acts as a molecular switch that controls fibre assembly. Nature 465:239–242. https://doi.org/10.1038/nature08936
Heiby JC, Rajab S, Rat C, Johnson CM, Neuweiler H (2017) Conservation of folding and association within a family of spidroin N-terminal domains. Sci Rep 7:16789. https://doi.org/10.1038/s41598-017-16881-6
Heiby JC, Goretzki B, Johnson CM, Hellmich, Ute A, Neuweiler H (2019) Methionine in a protein hydrophobic core drives tight interactions required for assembly of spider silk. Nat Commun 10:4378. https://doi.org/10.1038/s41467-019-12365-5
Jaudzems K, Askarieh G, Landreh M, Nordling K, Hedhammar M, Jörnvall H, Rising A, Knight SD, Johansson J (2012) pH-dependent dimerization of spider silk N-terminal domain requires relocation of a wedged tryptophan side chain. J Mol Biol 422:477–487. https://doi.org/10.1016/j.jmb.2012.06.004
Kronqvist N, Otikovs M, Chmyrov V, Chen G, Andersson M, Nordling K, Landreh M, Sarr M, Jörnvall H, Wennmalm S, Widengren J, Meng Q, Rising A, Otzen D, Knight SD, Jaudzems K, Johansson J (2014) Sequential pH-driven dimerization and stabilization of the N-terminal domain enables rapid spider silk formation. Nat Commun 5:3254. https://doi.org/10.1038/ncomms4254
Mulder FAA, Schipper D, Bott R, Boelens R (1999) Altered flexibility in the substrate-binding site of related native and engineered high-alkaline Bacillus subtilisins. J Mol Biol. https://doi.org/10.1006/jmbi.1999.3034
Ries J, Schwarze S, Johnson CM, Neuweiler H (2014) Microsecond folding and domain motions of a spider silk protein structural switch. J Am Chem Soc 136:17136–17144. https://doi.org/10.1021/ja508760a
Rising A, Johansson J (2015) Toward spinning artificial spider silk. Nat Chem Biol 11:309–315. https://doi.org/10.1038/nchembio.1789
Schwarze S, Zwettler FU, Johnson CM, Neuweiler H (2013) The N-terminal domains of spider silk proteins assemble ultrafast and protected from charge screening. Nat Commun 4:2815. https://doi.org/10.1038/ncomms3815
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223. https://doi.org/10.1007/s10858-009-9333-z
Vollrath F, Knight DP (2001) Liquid crystalline spinning of spider silk. Nature 410:541–548. https://doi.org/10.1038/35069000
Acknowledgements
BG acknowledges the Max Planck Graduate Centre (MPGC) for a PhD fellowship. HN acknowledges financial support from the U.S. Army Research Office (Grant Number W911NF-17-1-0336). UAH acknowledges support by the Carl Zeiss Foundation and the Center of Biomolecular Magnetic Resonance (BMRZ), Goethe University Frankfurt, funded by the state of Hesse.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Goretzki, B., Heiby, J.C., Hacker, C. et al. NMR assignments of a dynamically perturbed and dimerization inhibited N-terminal domain variant of a spider silk protein from E. australis. Biomol NMR Assign 14, 67–71 (2020). https://doi.org/10.1007/s12104-019-09922-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-019-09922-w