Abstract
Objective
To examine whether free (3-PD-5free) and/or liposomal (3-PD-5lipo) 6,7-dihydroxy-3-[3′,4′-methylenedioxyphenyl]-coumarin (3-PD-5) (1) modulate the effector functions of neutrophils from patients with rheumatoid arthritis under remission (i-RA) and with active disease (a-RA), in vitro; and (2) exert anti-inflammatory effect in a rat model of zymosan-induced acute joint inflammation.
Methods and results
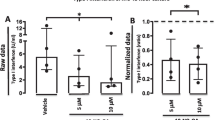
Incorporation of 3-PD-5 into unilamellar liposomes of soya phosphatidylcholine and cholesterol was efficient (57.5 ± 7.9%) and yielded vesicles with low diameter (133.7 ± 18.4 nm), polydispersity index (0.39 ± 0.06), and zeta potential (− 1.22 ± 0.34 mV). 3-PD-5free (1 µM) and 3-PD-5lipo (3 µM) equally suppressed elastase release and reactive oxygen species generation in neutrophils from healthy subjects and i-RA and a-RA patients, stimulated with immune complexes. 3-PD-5free (20 µM) suppressed the release of neutrophil extracellular traps and chemotaxis in vitro, without clear signs of cytotoxicity. 3-PD-5lipo (1.5 mg/kg, i.p.) diminished joint edema and synovial infiltration of total leukocytes and neutrophils, without changing the synovial levels of TNF-α, IL-1β, and IL-6.
Conclusion
Altogether, the results reported herein indicate that 3-PD-5 is a promising modulator of the early stages of acute joint inflammation that can help to diminish not only excessive neutrophil infiltration in the synovia but also neutrophil activation and its outcomes in RA patients.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- a-RA:
-
Active rheumatoid arthritis
- BTL1:
-
Leukotriene B4 receptor
- CL:
-
Chemiluminescence
- CXCL8:
-
Interleukin 8
- CXCR1:
-
CXCL8 receptor type 1
- CXCR2:
-
CXCL8 receptor type 2
- DAPI:
-
4′,6-Diamidino-2-phenylindole dihydrochloride
- DMSO:
-
Dimethyl sulfoxide
- DPI:
-
Diphenyleneiodonium chloride
- FITC:
-
Fluorescein isothiocyanate
- HBSS:
-
Hank’s balanced saline solution
- HBSS-gel:
-
Hank’s balanced saline solution supplemented with gelatin
- IC:
-
Immune complex
- i-RA:
-
Inactive rheumatoid arthritis
- i-IC:
-
Immobilized immune complexes
- IL-1β:
-
Interleukin 1β
- IL-6:
-
Interleukin 6
- LTB4 :
-
Leukotriene B4
- MFI:
-
Median fluorescence intensity
- MPO:
-
Myeloperoxidase
- NETs:
-
Neutrophil extracellular traps
- OVA:
-
Ovalbumin
- PBS:
-
Phosphate-buffered saline
- 3-PD-5:
-
6,7-Dihydroxy-3-[3′,4′-methylenedioxyphenyl]-coumarin
- 3-PD-5free :
-
Free form of 3-PD-5
- 3-PD-5lipo :
-
Liposomal form of 3-PD-5
- p-IC:
-
Precipitated immune complexes
- p-ICops:
-
Precipitated immune complexes opsonized with human serum
- PE:
-
Phycoerythrin
- RA:
-
Rheumatoid arthritis
- ROS:
-
Reactive oxygen species
- TNF-α:
-
Tumor necrosis factor α
References
Vermeren S, Karmakar U, Rossi AG. Immune complex-induced neutrophil functions: a focus on cell death. Eur J Clin Invest. 2018;48(Suppl. 2):e12948. https://doi.org/10.1111/eci.12948.
Paoliello-Paschoalato AB, Moreira MR, Azzolini AECS, Cavenaghi AA, Marzocchi-Machado CM, Donadi EA, et al. Activation of complement alternative pathway in rheumatoid arthritis: implications in peripheral neutrophils functions. Open Autoimmun J. 2011;3:1–9. https://doi.org/10.2174/1876894601103010001.
Paoliello-Paschoalato AB, Marchi LF, Andrade MF, Kabeya LM, Donadi EA, Lucisano-Valim YM. Fc-gamma and complement receptors and complement proteins in neutrophil activation in rheumatoid arthritis: contribution to pathogenesis and progression and modulation by natural products. Evid Based Complement Altern Med. 2015;2015:429878. https://doi.org/10.1155/2015/429878.
Bala A, Mondal C, Haldar PK, Khandelwal B. Oxidative stress in inflammatory cells of patient with rheumatoid arthritis: clinical efficacy of dietary antioxidants. Inflammopharmacology. 2017;25(6):595–607. https://doi.org/10.1007/s10787-017-0397-1.
Behnen M, Leschczyk C, Möller S, Batel T, Klinger M, Solbach W, et al. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcγRIIIB and Mac-1. J Immunol. 2014;193(4):1954–65. https://doi.org/10.4049/jimmunol.1400478.
Cecchi I, Rosa IA, Menegatti E, Roccatello D, Collantes-Estevez E, Lopez-Pedrera C, et al. Neutrophils: novel key players in rheumatoid arthritis. Current and future therapeutic targets. Autoimmun Rev. 2018;17(11):1138–49. https://doi.org/10.1016/j.autrev.2018.06.006.
Cascão R, Rosário HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev. 2010;9(8):531–5. https://doi.org/10.1016/j.autrev.2009.12.013.
Kinger M, Kumar S, Kumar V. Some important dietary polyphenolic compounds: an anti-inflammatory and immunoregulatory perspective. Mini Rev Med Chem. 2018;18(15):1270–82. https://doi.org/10.2174/1389557517666170208143410.
Kirsch G, Abdelwahab AB, Chaimbault P. Natural and synthetic coumarins with effects on inflammation. Molecules. 2016;21:1322. https://doi.org/10.3390/molecules21101322.
Nonose N, Pereira JA, Machado PRM, Rodrigues MR, Sato DT, Martinez CAR. Oral administration of curcumin (Curcuma longa) can attenuate the neutrophil inflammatory response in zymosan-induced arthritis in rats. Acta Cir Bras. 2014;29(11):727–34. https://doi.org/10.1590/S0102-86502014001800006.
Figueiredo-Rinhel ASG, Andrade MF, Landi-Librandi AP, Azzolini AECS, Kabeya LM, Bastos JK, et al. Incorporation of Baccharis dracunculifolia DC (Asteraceae) leaf extract into phosphatidylcholine-cholesterol liposomes improves its anti-inflammatory effect in vivo. Nat Prod Res. 2018. https://doi.org/10.1080/14786419.2018.1448809.
Oliveira FFB, Araújo JCB, Pereira AF, Brito GAC, Gondim DV, Ribeiro RA, et al. Antinociceptive and anti-inflammatory effects of Caryocar coriaceum Wittm fruit pulp fixed ethyl acetate extract on zymosan-induced arthritis in rats. J Ethnopharmacol. 2015;174:452–63. https://doi.org/10.1016/j.jep.2015.08.017.
Vongsak B, Gritsanapan W, Wongkrajang Y, Jantan I. In vitro inhibitory effects of Moringa oleifera leaf extract and its major components on chemiluminescence and chemotactic activity of phagocytes. Nat Prod Commun. 2013;8(11):1559–611. https://doi.org/10.1177/1934578X1300801115.
Detsi A, Kontogiorgis C, Hadjipavlou-Litina D. Coumarin derivatives: an updated patent review (2015–2016). Expert Opin Ther Pat. 2017;27(11):1201–26. https://doi.org/10.1080/13543776.2017.1360284.
Kabeya LM, Marchi AA, Kanashiro A, Lopes NP, Silva CHTP, Pupo MT, et al. Inhibition of horseradish peroxidase catalytic activity by new 3-phenylcoumarin derivatives: synthesis and structure-activity relationships. Bioorg Med Chem. 2007;15(3):1516–24. https://doi.org/10.1016/j.bmc.2006.10.068.
Roussaki M, Kontogiorgis CA, Hadjipavlou-Litina D, Hamilakis S, Detsi A. A novel synthesis of 3-aryl coumarins and evaluation of their antioxidant and lipoxygenase inhibitory activity. Bioorg Med Chem Lett. 2010;20(13):3889–922. https://doi.org/10.1016/j.bmcl.2010.05.022.
Robledo-O'Ryan N, Matos MJ, Vazquez-Rodriguez S, Santana L, Uriarte E, Moncada-Basualto M, et al. Synthesis, antioxidant and antichagasic properties of a selected series of hydroxy-3-arylcoumarins. Bioorg Med Chem. 2017;25(2):621–32. https://doi.org/10.1016/j.bmc.2016.11.033.
Kabeya LM, Silva CHTP, Kanashiro A, Campos JM, Azzolini AECS, Polizello ACM, et al. Inhibition of immune complex-mediated neutrophil oxidative metabolism: a pharmacophore model for 3-phenylcoumarin derivatives using GRIND-based 3D-QSAR and 2D-QSAR procedures. Eur J Med Chem. 2008;43(5):996–1007. https://doi.org/10.1016/j.ejmech.2007.07.003.
Andrade MF, Kabeya LM, Azzolini AECS, Santos EOL, Figueiredo-Rinhel ASG, Paris MRP, et al. 3-Phenylcoumarin derivatives selectively modulate different steps of reactive oxygen species production by immune complex-stimulated human neutrophils. Int Immunopharmacol. 2013;15(2):387–94. https://doi.org/10.1016/j.intimp.2013.01.001.
Andrade MF, Kabeya LM, Bortot LO, Santos GB, Santos EOL, Albiero LR, et al. The 3-phenylcoumarin derivative 6,7-dihydroxy-3–3',4'-methylenedioxyphenyl-coumarin downmodulates the FcγR- and CR-mediated oxidative metabolism and elastase release in human neutrophils: possible mechanisms underlying inhibition of the formation and release of neutrophil extracellular traps. Free Radic Biol Med. 2018;115:421–35. https://doi.org/10.1016/j.freeradbiomed.2017.12.012.
Landi-Librandi AP, Chrysostomo TN, Azzolini AECS, Marzocchi-Machado CM, de Oliveira CA, Lucisano-Valim YM. Study of quercetin-loaded liposomes as potential drug carriers: in vitro evaluation of human complement activation. J Liposome Res. 2012;22(2):89–99. https://doi.org/10.3109/08982104.2011.615321.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Frie JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. https://doi.org/10.1002/art.1780310302.
Dougados M, Aletaha D, van Riel P. Disease activity measures for rheumatoid arthritis. Clin Exp Rheumatol. 2007;25(5 Suppl 46):S22–S29.
Paoliello-Paschoalato AB, Azzolini AECS, Cruz MFC, Marchi LF, Kabeya LM, Donadi EA, et al. Isolation of healthy individuals' and rheumatoid arthritis patients' peripheral blood neutrophils by the gelatin and Ficoll-Hypaque methods: comparative efficiency and impact on the neutrophil oxidative metabolism and Fe gamma receptor expression. J Immunol Methods. 2014;412:70–7. https://doi.org/10.1016/j.jim.2014.07.001.
Santos EOL, Azzolini AECS, Lucisano-Valim YM. Optimization of a flow cytometric assay to evaluate the human neutrophil ability to phagocytose immune complexes via Fcγ and complement receptors. J Pharmacol Toxicol Methods. 2015;72:67–71. https://doi.org/10.1016/j.vascn.2014.10.005.
Lucisano YM, Mantovani B. Lysosomal enzyme release from polymorphonuclear leukocytes induced by immune complexes of IgM and IgG. J Immunol. 1984;132(4):2015–20.
Lucisano-Valim YM, Spadaro ACC. Isolation of gamma-globulin (IgG) from serum: a task that encourages students to consolidate concepts on protein structure. Biochem Educ. 1998;26(1):63–5. https://doi.org/10.1016/S0307-4412(97)00121-0.
Souto FO, Zarpelon AC, Staurengo-Ferrari L, Fattori V, Casagrande R, Fonseca MJV, et al. Quercetin reduces neutrophil recruitment induced by CXCL8, LTB4, and fMLP: inhibition of actin polymerization. J Nat Prod. 2011;74(2):113–8. https://doi.org/10.1021/np1003017.
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow-cytometry. J Immunol Methods. 1991;139(2):271–9. https://doi.org/10.1016/0022-1759(91)90198-O.
Kanashiro A, Pessini AC, Machado RR, Malvar DC, Aguiar FA, Soares DM, et al. Characterization and pharmacological evaluation of febrile response on zymosan-induced arthritis in rats. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1631–R16401640. https://doi.org/10.1152/ajpregu.90527.2008.
Marchi LF, Paoliello-Paschoalato AB, Oliveira RDR, Azzolini AECS, Kabeya LM, Donadi EA, et al. Activation status of peripheral blood neutrophils and the complement system in adult rheumatoid arthritis patients undergoing combined therapy with infliximab and methotrexate. Rheumatol Int. 2018;38(6):1043–52. https://doi.org/10.1007/s00296-018-3997-1.
Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17(3):638–50. https://doi.org/10.1016/j.intimp.2013.06.034.
Albiero LR, Andrade MF, Marchi LF, Azzolini AECS, Oliveira RDR, Emery FS, et al. Therapeutic potential modulation of 3-phenylcoumarin derivative-loaded liposomes in peripheral blood neutrophils functions from rheumatoid arthritis patients. CD-ROM. Annals of XLI Congress of the Brazilian Society of Immunology; 2016 Oct 29-Nov 2; Campos do Jordão, SP (Brazil): SBI; c2016. CD-ROM.
Fairhurst AM, Wallace PK, Jawad ASM, Goulding NJ. Rheumatoid peripheral blood phagocytes are primed for activation but have impaired Fc-mediated generation of reactive oxygen species. Arthritis Res Ther. 2007;9(2):R29. https://doi.org/10.1186/ar2144.
Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, et al. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. PNAS. 2010;107(8):3546–51. https://doi.org/10.1073/pnas.0914351107.
Sakai J, Li J, Subramanian KK, Mondal S, Bajrami B, Hattori H, et al. Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity. 2012;37(6):1037–49. https://doi.org/10.1016/j.immuni.2012.08.017.
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–91. https://doi.org/10.1083/jcb.201006052.
Reeves EP, Lu H, Jacobs HL, Messina CGM, Bolsover S, Gabella G, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416(6878):291–7. https://doi.org/10.1038/416291a.
Kawakami E, Tokunaga A, Ozawa M, Sakamoto R, Yoshida N. The histone demethylase Fbxl11/Kdm2a plays an essential role in embryonic development by repressing cell-cycle regulators. Mech Dev. 2015;135:31–42. https://doi.org/10.1016/j.mod.2014.10.001.
Kirchner T, Möller S, Klinger M, Solbach W, Laskay T, Behnen M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediat Inflamm. 2012;2012:849136. https://doi.org/10.1155/2012/849136.
Lightfoot YL, Kaplan MJ. Disentangling the role of neutrophil extracellular traps in rheumatic diseases. Curr Opin Rheumatol. 2017;29(1):65–70. https://doi.org/10.1097/BOR.0000000000000357.
Pérez-Sánchez C, Ruiz-Limón P, Aguirre MA, Jiménez-Gómez Y, Arias-de la Rosa I, Ábalos-Aguilera MC, et al. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in rheumatoid arthritis patients. J Autoimmun. 2017;82:31–40. https://doi.org/10.1016/j.jaut.2017.04.007.
Nanjundaiah SM, Astry B, Moudgil KD. Mediators of inflammation-induced bone damage in arthritis and their control by herbal products. Evid Based Complement Altern Med. 2013;2013:518094. https://doi.org/10.1155/2013/518094.
Munin A, Edwards-Lévy F. Encapsulation of natural polyphenolic compounds: a review. Pharmaceutics. 2011;3(4):793–829. https://doi.org/10.3390/pharmaceutics3040793.
van den Hoven JM, Van Tomme SR, Metselaar JM, Nuijen B, Beijnen JH, Storm G. Liposomal drug formulations in the treatment of rheumatoid arthritis. Mol Pharm. 2011;8(4):1002–155. https://doi.org/10.1021/mp2000742.
Kapoor B, Singh SK, Gulati M, Gupta R, Vaidya Y. Application of liposomes in treatment of rheumatoid arthritis: quo vadis. Sci World J. 2014;2014:978351. https://doi.org/10.1155/2014/978351.
Jaafar-Maalej C, Diab R, Andrieu V, Elaissari A, Fessi H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J Liposome Res. 2010;20(3):228–43. https://doi.org/10.3109/08982100903347923.
Rzodkiewicz P, Gąsińska E, Gajewski M, Bujalska-Zadrożny M, Szukiewicz D, Maśliński S. Esculetin reduces leukotriene B4 level in plasma of rats with adjuvant-induced arthritis. Reumatologia. 2016;54(4):161–4. https://doi.org/10.5114/reum.2016.62469.
Umar S, Kumar A, Sajad M, Zargan J, Ansari M, Ahmad S, et al. Hesperidin inhibits collagen-induced arthritis possibly through suppression of free radical load and reduction in neutrophil activation and infiltration. Rheumatol Int. 2013;33(3):657–63. https://doi.org/10.1007/s00296-012-2430-4.
Gardi C, Bauerova K, Stringa B, Kuncirova V, Slovak L, Ponist S, et al. Quercetin reduced inflammation and increased antioxidant defense in rat adjuvant arthritis. Arch Biochem Biophys. 2015;583:150–7. https://doi.org/10.1016/j.abb.2015.08.008.
Landi-Librandi AP, Oliveira CA, Azzolini AECS, Kabeya LM, del Ciampo JO, Bentley MVLB, et al. In vitro evaluation of the antioxidant activity of liposomal flavonols by the HRP-H2O2-luminol system. J Microencapsul. 2011;28(4):258–67. https://doi.org/10.3109/02652048.2011.559283.
Landi-Librandi AP, Azzolini AECS, Oliveira CA, Lucisano-Valim YM. Inhibitory activity of liposomal flavonoids during oxidative metabolism of human neutrophils upon stimulation with immune complexes and phorbol ester. Drug Deliv. 2012;19(4):177–87. https://doi.org/10.3109/10717544.2012.679710.
Acknowledgments
The authors thank Dr. Adriana Balbina Paoliello-Paschoalato, Dr. Marcelo Dias Baruffi and Dr. Alexandre Kanashiro for scientific discussions, and Mr. Alcides Silva Pereira and Mrs. Nadir Mazzucato from the School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Brazil, for their helpful technical assistance.
Funding
This study was supported by the Brazilian funding agencies São Paulo Research Foundation (FAPESP, grants 2010/19504-0, 2012/23541-4, 2013/20810-7, and 2013/21331-5) and National Council for Scientific and Technological Development (CNPq, grants 482015/2012-8, 308111/2013-3, and 130209/2015-5).
Author information
Authors and Affiliations
Contributions
LRA, MFA, LFM, and YMLV conceived the study; LRA, MFA, LFM, and AECS designed the experiments; RDRO selected the patients and determined their clinical parameters; LRA and LFM selected the healthy volunteers; LRA, MFA, CAC, and AECS performed the in vitro experiments; MFA, APLL, ASGFR, and AECS performed the in vivo experiments; LRA, MFA, LFM, LMK, AECS, and YMLV analyzed the data and discussed the results; MTP and FSE synthesized the 3-phenylcoumarin derivative and discussed the results; LRA, MFA, and LMK wrote the manuscript; and YMLV searched for funding and supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval and consent to participate
All procedures performed in studies involving human participants complied with the ethical standards established by the Resolution 466/2012 of the Brazilian National Health Council, and with the 1964 Helsinki declaration and its later amendments. The Human Research Ethics Committee from the Ribeirão Preto Medical School Hospital and from the School of Pharmaceutical Sciences of Ribeirão Preto, both at the University of São Paulo, Ribeirão Preto, SP, Brazil, approved the study protocol (CEP/HCRP n. 15038/2015). All the participants signed an informed consent form to participate in this study. All procedures performed in studies involving animals complied with the ethical standards of The Animal Care Committee from the University of São Paulo, Brazil, which approved the study protocol (CEUA n. 15.1.1163.60.0).
Additional information
Responsible Editor: Jason J. McDougall.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Albiero, L.R., de Andrade, M.F., Marchi, L.F. et al. Immunomodulating action of the 3-phenylcoumarin derivative 6,7-dihydroxy-3-[3′,4′-methylenedioxyphenyl]-coumarin in neutrophils from patients with rheumatoid arthritis and in rats with acute joint inflammation. Inflamm. Res. 69, 115–130 (2020). https://doi.org/10.1007/s00011-019-01298-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01298-w