Abstract
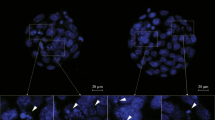
The frequency of congenital malformations is 3–5 times higher in mothers with pregestational diabetes mellitus than in general population. Apparently, this problem is due to change in the expression of apoptotic and antiapoptotic genes induced by the oxidative stress derived from the diabetes/hyperglycemia. One of these genes is Bcl-2, which is associated with the control and inhibition of apoptosis. The purpose of the present work was to study the effect of polyamine addition over expression of Bcl-2 gene in a model of diabetic embryopathy. For this, gestational day 10.5 (GD10.5) rat embryos were incubated at 37°C for 24 h in control medium, medium with high glucose, or medium with high glucose and supplemented with spermidine or spermine. Post-cultured embryos were harvested and observed to obtain morphological scores; some of them were subjected to molecular biology studies: DNA isolation plus conventional PCR or RNA isolation plus RT-PCR; other embryos were fixed with paraformaldehyde and used for immunohistochemical detection of Bcl-2 protein. Although Bcl-2 mRNA was similarly expressed in all rat embryo treatments, Bcl-2 protein was found only in control-incubated embryos. In conclusion, it seems that the inhibition of Bcl-2 gene expression induced by glucose was not reversed by polyamines.




Similar content being viewed by others
References
Baack ML, Wang C, Hu S, Segar JL, Norris AW (2014) Hyperglycemia induces embryopathy, even in the absence of systemic maternal diabetes: an in vivo test of the fuel mediated teratogenesis hypothesis. Reprod Toxicol 46:129–136
Bellé NAV, Dalmolin GD, Fonini G, Rubin MA, Rocha JB (2004) Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res 1008(2):245–251
Bredow S, Juri D, Cardin K, Tesfaigzi Y (2007) Identification of a novel Bcl-2 promoter region that counteracts in a p53-dependent manner the inhibitory P2 region. Gene 404:110–116
Brownlee M (2002) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Calciu C, Kubow S, Chan H (2002) Interactive dysmorphogenic effects of toxaphene or toxaphene congeners and hyperglycemia on cultured whole rat embryos during organogenesis. Toxicology 175:153–165
Cederberg J, Galli J, Luthman H, Eriksson U (2000) Increased mRNA levels of Mn-SOD and catalase in embryos of diabetic rats from a malformation resistant strain. Diabetes 49:101–107
Chappel JH, Wamg XD, Loeken MR (2009) Diabetes and apoptosis: neural crest cells and neural tube. Apoptosis 14:1472–1483
Chattopadhyay MK, Tabor CW, Tabor H (2003) Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc Natl Acad Sci U S A 100:2261–2265
Childs AC, Mehta DJ, Gerner EW (2003) Polyamine-dependent gene expression. Cell Mol Life Sci 60:1394–1406
Chirino-Galindo G, Baiza-Gutman LA, Barrera-Escorcia E, Palomar-Morales M (2009) Polyamines protect rat embryo in vitro from high glucose-induced developmental delay and dysmorphogenesis. Birth Def Res B Dev Reprod Toxicol 86:58–64
Chirino-Galindo G, Mejía R, Palomar-Morales M (2012) Change in lipoperoxidation but not in scavenging enzymes activity during polyamine embryoprotection in rat embryo cultured in hyperglycemic media. In Vitro Cell Dev Biol Anim 48:570–576
Clapés S, Fernández T, Suárez G (2013) Oxidative stress and birth defects in infants of women with pregestational diabetes. MEDICC Rev 15:37–40
ENSANUT (2016) Encuesta nacional de salud y nutrición de medio camino. INSP México, pp 46–53
Eriksson U, Cederberg J, Wentzel P (2003) Congenital malformations in offspring of diabetic mothers - Animal and human studies. Rev Endocr Metab Disord 4:79–93
Eriksson U, Hakan B, Forsberg H, Styrud J (1991) Diabetic embryopathy: studies with animal and in vitro models. Diabetes 40:94–98
Eriksson UF, Wentzel P (2016) The status of diabetic embryopathy. Ups J Med Sci 121:96–112
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (2003) Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 25:s5–s20
Farrell RE Jr (2010) RNA Methodologies. Laboratory guide for insolation and characterization, 4th edn. Academic Press, Amsterdam San Diego
Forsberg H, Borg L, Cagliero E, Eriksson U (1996) Altered levels of scavenging enzymes in embryos subjected to a diabetic environment. Free Radic Res 24:451–459
Forsberg H, Eriksson J, Welsh N (1998) Apoptosis in embryos of diabetic rats. Pharmacol Toxicol 83:104–111
Fozard JR, Prat ML, Prakash NJ, Grove J, Schechter PJ, Sjoerdsma A, Koch J (1980) L-Ornithine decarboxylase: an essential role in early mammalian embryogenesis. Science 208:505–509
Freinkel N (1980) The Banting lecture: of pregnancy and progeny. Diabetes 29:1023–1035
Fujinaga M, Baden JM (1992) Variation in development of rat embryos at the presomite period. Teratology 45:661–670
Gäreskog M, Cederberg J, Eriksson U, Wentzel P (2007) Maternal diabetes in vivo and high glucose concentration in vitro increases apoptosis in rat embryos. Reprod Toxicol 23:63–74
Goldman A, Baker L, Piddington R, Marx B, Herold R, Egler J (1985) Hyperglicemia-induced teratogenesis is mediated by a functional deficiency of arachidonic acid. Proc Natl Acad Sci U S A 82:8227–8231
Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA (1998) The natural polyamine spermine functions directly as free radical scavenger. Proc Natl Acad Sci U S A 95:11140–11145
Hinojosa M, Hernández F, Barrera E, Gayosso M (2010) Prevalencia de Diabetes mellitus gestacional en el Hospital Juárez de México. Rev Hosp Jua Méx 77:123–128
Hussain T, Tan B, Ren W, Rahu N, Kalhoro D, Yin Y (2017) Exploring polyamines: functions in embryo/fetal development. Anim Nutr 3:7–10
Klug S, Lewandowski C, Neubert D (1985) Modification and standardization of the culture of early postimplantation embryos for toxicological studies. Arch Toxicol 58:84–88
Kruger A, Peterson S, Schwartzman M, Fusco H, McClung J, Weiss M, Shenouda S, Goodman A, Goligorsky M, Kappas A, Nader A (2006) Up-regulation of heme oxygenase provides vascular protection in an animal model of Diabetes through Its antioxidant and antiapoptotic effects. J Pharmacol Exp Ther 319:1144–1152
Méndez JD, Palomar-Morales M (1999) Prevention by L-arginine and polyamines of delayed development and embryotoxicity caused by chemically-induced diabetes in rats. Reprod Toxicol 13:501–509
Mendoza A, Monroy G, Morimoto S, Cerbón M (2003) Pro-apoptotic signals of the Bcl-2 gene family in the rat uterus occurs in the night before the day of estrus and precedes ovulation. Mol Cell Endocrinol 208:31–39
Metcalfe AD, Hunter HR, Bloor D (2004) Expression of 11 members of the BCL-2 family of apoptosis regulatory molecules during human preimplantation embryo development and fragmentation. Mol Reprod Dev 68:35–50
Miller-Fleming L, Olin-Sandoval V, Campbell K, Ralser M (2015) Remaining mysteries of molecular biology: The role of polyamines in the cell. J Mol Biol 427:3389–3406
New DAT (1978) Whole-embryo culture and the study of mammalian embryos during organogenesis. Biol Rev Camb Philos Soc 53:81–122
Ornoy A (2007) Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol 24:31–41
Suhonen L, Hiilesmaa V, Teramo K (2000) Glycaemic control during early pregnancy and fetal malformations in women with type I Diabetes mellitus. Obstet Gynecol Surv 55:474–476
Sun F, Akazawa S, Kazuyuki S, Kamihira S, Kawasaki E, Eguchi K, Koji T (2002) Apoptosis is normal rat embryo tissues during early organogenesis: The possible Involvement of Bax and Bcl-2. Arch Cytol Histol 65:145–157
Sun F, Kawasaki E, Akazawa S, Hishikawa Y, Sugahara K, Kamihira S, Koji T, Eguchi K (2005) Apoptosis and its pathway in early post-implantation embryos of diabetic rats. Diabet Res Clin Pract 67:110–118
Wentzel P, Eriksson U (2005) A diabetes-like environment increases malformation rate and diminishes prostaglandin E2 in rat embryos: reversal by administration of vitamin E and folic acid. Birth Def Res Part A Clin Mol Teratol 73:506–511
Wentzel P, Eriksson U (2011) Altered gene expression in rat cranial neural crest cells exposed to a teratogenic glucose concentration in vitro-paradoxical downregulation of antioxidative defense genes. Birth Def Res B Dev Reprod Toxicol 92:487–497
Yang P, Zhao Z, Reece EA (2008) Blockade of c-Jun N-terminal kinase activation abrogates hyperglycemia-induced yolk sac vasculopathy in vitro. Am J Obstet Gynecol 198(321):e1–e7
Zabihi S, Eriksson UJ, Wentzel P (2007) Folic acid supplementation affects ROS scavenging enzymes, enhances Vegf-A, and diminishes apoptotic state in yolk sacs of embryos of diabetic rats. Reprod Toxicol 23:486–498
Acknowledgments
M. Sc. Fernando Barrón Moreno (Bioterio, FESI) helps to authors in the care and maintenance of experimental subjects. M. Sc. Alejandro C. Monsalvo (Sequentiation Unit, UBIPRO) made the sequentiation of the PCR products. Authors should acknowledge to P. de B. Liliana Berenice Ramírez Domínguez for grammar revision, and DG Raquel Flores Olmedo for image edition.
Funding
This work was partially supported by PAPIIT (IN215414) from DGAPA, UNAM to MPM; and PAPCA 2016 (Project FESI-DIP-PAPCA-2016-17), from the División de Investigación y Posgrado, FES Iztacala, UNAM, to MPM and RMZ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Chirino-Galindo, G., Hernández-Hernández, D.E., Reyes-Mateos, L.C. et al. Bcl-2 expression in a diabetic embryopathy model in presence of polyamines. In Vitro Cell.Dev.Biol.-Animal 55, 821–829 (2019). https://doi.org/10.1007/s11626-019-00400-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-019-00400-0