Abstract
Objective
This systematic review and meta-analysis aimed to provide evidence-based guidance to better understand the risk of central line-associated bloodstream infection (CLABSI) in cancer patients who received totally implantable venous access ports (TIVAPs) compared with those who received external central venous catheters (CVCs).
Methods
A systematic search of PubMed, Web of science, Embase, and the Cochrane Library was carried out from inception through Oct 2018, with no language restrictions. Trials examining the risk of CLABSI in cancer patients who received TIVAPs compared with those who received external CVCs were included. Two reviewers independently reviewed, extracted data, and assessed the risk of bias of each study. A random-effect model was used to estimate relative risks (RRs) with 95% CIs.
Results
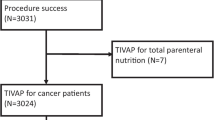
In all, 26 studies involving 27 cohorts and 5575 patients reporting the incidence of CLABSI in patients with TIVAPs compared with external CVCs were included. Pooled meta-analysis of these trials revealed that TIVAPs were associated with a significant lower risk of CLABSI than were external CVCs (relative risk [RR], 0.44; 95% confidence interval [CI], 0.31–0.62; P < 0.00001), which was confirmed by trial sequential analysis for the cumulative z curve entered the futility area. Subgroup analyses demonstrated that CLABSI reduction was greatest in adult patients (RR [95% CI], 0.35 [0.22–0.56]) compared with pediatric patients who received TIVAPs (RR [95% CI], 0.55 [0.38–0.79]).
Conclusions
TIVAP can significantly reduce the risk of CLABSI compared with external CVCs.



Similar content being viewed by others
References
Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E (1982) Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery 92(4):706–712
Kurul S, Saip P, Aydin T (2002) Totally implantable venous-access ports: local problems and extravasation injury. Lancet Oncol 3(11):684–692
iData Research. U.S. Markets for Vascular Access Devices and Accessories (2012) Vancouver. In: BC: iData research
Pinelli F, Cecero E, Degl'Innocenti D et al (2018) Infection of totally implantable venous access devices: a review of the literature. J Vasc Access 19(3):230–242
Lebeaux D, Fernández-Hidalgo N, Chauhan A, Lee S, Ghigo JM, Almirante B, Beloin C (2014) Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis 14(2):146–159
Fang S, Yang J, Song L, Jiang Y, Liu Y (2017) Comparison of three types of central venous catheters in patients with malignant tumor receiving chemotherapy. Patient Prefer Adherence 11:1197–1204
Wu O, Boyd K, Paul J, McCartney E, Ritchie M, Mellon D, Kelly L, Dixon-Hughes J, Moss J (2016) Hickman catheter and implantable port devices for the delivery of chemotherapy: a phase II randomized controlled trial and economic evaluation. Br J Cancer 114(9):979–985
Lefebvre L, Noyon E, Georgescu D, Proust V, Alexandru C, Leheurteur M, Thery JC, Savary L, Rigal O, di Fiore F, Veyret C, Clatot F (2016) Port catheter versus peripherally inserted central catheter for postoperative chemotherapy in early breast cancer: a retrospective analysis of 448 patients. Support Care Cancer 24(3):1397–1403
Patel GS, Jain K, Kumar R, Strickland AH, Pellegrini L, Slavotinek J, Eaton M, McLeay W, Price T, Ly M, Ullah S, Koczwara B, Kichenadasse G, Karapetis CS (2014) Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer 22(1):121–128
Beckers MM, Ruven HJ, Seldenrijk CA, Prins MH, Biesma DH (2010) Risk of thrombosis and infections of central venous catheters and totally implanted access ports in patients treated for cancer. Thromb Res 125(4):318–321
Pegues D, Axelrod P, McClarren C, Eisenberg BL, Hoffman JP, Ottery FD, Keidan RD, Boraas M, Weese J (1992) Comparison of infections in Hickman and implanted port catheters in adult solid tumor patients. J Surg Oncol 49(3):156–162
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52(6):377–384
Sweeting MJ, Sutton AJ, Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23(9):1351–1375
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Wetterslev J, Thorlund K, Brok J, Gluud C (2008) Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 61(1):64–75
Brok J, Thorlund K, Wetterslev J, Gluud C (2009) Apparently conclusive meta-analyses may be inconclusive--trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 38(1):287–298
Brok J, Thorlund K, Gluud C, Wetterslev J (2008) Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 61(8):763–769
Handrup MM, Moller JK, Frydenberg M, Schroder H (2010) Placing of tunneled central venous catheters prior to induction chemotherapy in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 55(2):309–313
Zganjer M, Cizmic A, Butkovic D et al (2008) Central venous catheters for chemotherapy of solid tumors--our results in the last 5 years. Coll Antropol 32(3):767–770
Ng F, Mastoroudes H, Paul E, Davies N, Tibballs J, Hochhauser D, Mayer A, Begent R, Meyer T (2007) A comparison of Hickman line- and port-a-Cath-associated complications in patients with solid tumours undergoing chemotherapy. Clin Oncol (R Coll Radiol) 19(7):551–556
Adler A, Yaniv I, Steinberg R et al (2006) Infectious complications of implantable ports and Hickman catheters in paediatric haematology-oncology patients. J Hosp Infect 62(3):358–365
Johansson E, Bjorkholm M, Bjorvell H et al (2004) Totally implantable subcutaneous port system versus central venous catheter placed before induction chemotherapy in patients with acute leukaemia-a randomized study. Support Care Cancer 12(2):99–105
Dillon PW, Jones GR, Bagnall-Reeb HA, Buckley JD, Wiener ES, Haase GM, Children's Oncology Group (2004) Prophylactic urokinase in the management of long-term venous access devices in children: a Children's oncology group study. J Clin Oncol 22(13):2718–2723
Pracchia LF, Dias LC, Dorlhiac-Llacer PE, Chamone DA (2004) Comparison of catheter-related infection risk in two different long-term venous devices in adult hematology-oncology patients. Rev Hosp Clin Fac Med Sao Paulo 59(5):291–295
Estes JM, Rocconi R, Straughn JM et al (2003) Complications of indwelling venous access devices in patients with gynecologic malignancies. Gynecol Oncol 91(3):591–595
Minassian VA, Sood AK, Lowe P, Sorosky JI, al-Jurf AS, Buller RE (2000) Longterm central venous access in gynecologic cancer patients. J Am Coll Surg 191(4):403–409
Silver DF, Hempling RE, Recio FO, Piver MS, Eltabbakh GH (1998) Complications related to indwelling caval catheters on a gynecologic oncology service. Gynecol Oncol 70(3):329–333
Eastridge BJ, Lefor AT (1995) Complications of indwelling venous access devices in cancer patients. J Clin Oncol 13(1):233–238
Gleeson NC, Fiorica JV, Mark JE, Pinelli DM, Hoffman MS, Roberts WS, Cavanagh D (1993) Externalized Groshong catheters and Hickman ports for central venous access in gynecologic oncology patients. Gynecol Oncol 51(3):372–376
Groeger JS, Lucas AB, Thaler HT, Friedlander-Klar H, Brown AE, Kiehn TE, Armstrong D (1993) Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Ann Intern Med 119(12):1168–1174
Mueller BU, Skelton J, Callender DP, Marshall D, Gress J, Longo D, Norton J, Rubin M, Venzon D, Pizzo PA (1992) A prospective randomized trial comparing the infectious and noninfectious complications of an externalized catheter versus a subcutaneously implanted device in cancer patients. J Clin Oncol 10(12):1943–1948
Wacker P, Bugmann P, Halperin DS, Babel JF, le Coultre C, Wyss M (1992) Comparison of totally implanted and external catheters in paediatric oncology patients. Eur J Cancer 28A(4–5):841–844
Kappers-Klunne MC, Degener JE, Stijnen T, Abels J (1989) Complications from long-term indwelling central venous catheters in hematologic patients with special reference to infection. Cancer -Am Cancer Soc 64(8):1747–1752
Carde P, Cosset-Delaigue MF, Laplanche A, Chareau I (1989) Classical external indwelling central venous catheter versus totally implanted venous access systems for chemotherapy administration: a randomized trial in 100 patients with solid tumors. Eur J Cancer Clin Oncol 25(6):939–944
Greene FL, Moore W, Strickland G, McFarland J (1988) Comparison of a totally implantable access device for chemotherapy (port-A-Cath) and long-term percutaneous catheterization (Broviac). South Med J 81(5):580–583
Ross MN, Haase GM, Poole MA, Burrington JD, Odom LF (1988) Comparison of totally implanted reservoirs with external catheters as venous access devices in pediatric oncologic patients. Surg Gynecol Obstet 167(2):141–144
Wurzel CL, Halom K, Feldman JG, Rubin LG (1988) Infection rates of Broviac-Hickman catheters and implantable venous devices. Am J Dis Child 142(5):536–540
Penel N, Neu J, Clisant S et al (2007) Risk factors for early catheter-related infections in cancer patients. Cancer -Am Cancer Soc 110(7):1586–1592
Fratino G, Molinari AC, Parodi S, Longo S, Saracco P, Castagnola E, Haupt R (2005) Central venous catheter-related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol 16(4):648–654
Mirro JJ, Rao BN, Stokes DC et al (1989) A prospective study of Hickman/Broviac catheters and implantable ports in pediatric oncology patients. J Clin Oocol 7(2):214–222
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJA, Sherertz RJ, Warren DK (2009) Clinical practice guidelines for the diagnosis and Management of Intravascular Catheter-Related Infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49(1):1–45
Funding
This work was supported by grants from the President Foundation of Nanfang Hospital of Southern Medical University (Grant No.2016B012) and grants from President Foundation of Nanfang Hospital, Southern Medical University (CX2018NO13).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests. We have full control of all primary data and agree to allow the journal to review our data if requested.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Test for publication bias. (PNG 77 kb)
Rights and permissions
About this article
Cite this article
Jiang, M., Li, Cl., Pan, Cq. et al. The risk of bloodstream infection associated with totally implantable venous access ports in cancer patient: a systematic review and meta-analysis. Support Care Cancer 28, 361–372 (2020). https://doi.org/10.1007/s00520-019-04809-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04809-x