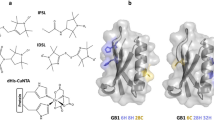
Abstract
Site-directed spin labeling of proteins by chemical modification of engineered cysteine residues with the molecule MTSSL (1-oxyl-2,2,5,5-tetramethylpyrroline-3-methyl methanethiosulfonate) has been an invaluable tool for conducting double electron electron resonance (DEER) spectroscopy experiments. However, this method is generally limited to recombinant proteins with a limited number of reactive Cys residues that when modified will not impair protein function. Here, we present a method that allows for spin labeling of protein-nucleotide-binding sites by adenosine diphosphate (ADP) modified with a nitroxide moiety on the β-phosphate (ADP-β-S-SL). The synthesis of this ADP analog is straightforward and isolation of pure product is readily achieved on a standard reverse-phase high-performance liquid chromatography (HPLC) system. Furthermore, analyses of isolated ADP-β-S-SL by LC–mass spectrometry confirm that the molecule is very stable under ambient conditions. The crystal structure of ADP-β-S-SL bound to the ATP pocket of the histidine kinase CheA reveals specific targeting of the probe, whose nitroxide moiety is mobile on the protein surface. Continuous wave and pulsed-ESR measurements demonstrate the capability of ADP-β-S-SL to report on active site environment and provide reliable DEER distance constraints.




Similar content being viewed by others
References
G. Jeschke, Interpretation of dipolar EPR data in terms of protein structure. Structural Information from Spin-Labels and Intrinsic Paramagnetic Centres in the Biosciences, ed. by C.R. Timmel, J.R. Harmer (Springer, Berlin, 2013), pp. 83–120
J.P. Klare, Biol. Chem. 394, 1281–1300 (2013)
P. Borbat, J. Freed, eMagRes 6, 465–493 (2017). https://doi.org/10.1002/9780470034590.emrstm1519
W.L. Hubbell, A. Gross, R. Langen, M.A. Lietzow, Curr. Opin. Struct. Biol. 8, 649–656 (1998)
C. Gmeiner, D. Klose, E. Mileo, V. Belle, S. Marque, G. Dorn, F. Allain, B. Guigliarelli, G. Jeschke, M. Yulikov, J. Phys. Chem. Lett. 8, 4852–4857 (2017)
C. Bertrand, E.M. Bruch, P. Demay-drouhard, R.M. Rasia, C. Policar, L.C. Tabares, J. Phys. Chem. Lett. 7, 1072–1076 (2016)
M.J. Schmidt, J. Borbas, M. Drescher, D. Summerer, J. Am. Chem. Soc. 3, 1238–1241 (2014)
M.R. Fleissner, E.M Brustad, T. Kaki, C. Attenbach, D. Cascio, F.B. Peters, K. Hides, S. Peuker, P.G. Schultz, W.L. Hubbell, Proc. Natl. Acad. USA 106, 21637–21642 (2009)
A.J. Fielding, M.G. Concilio, G. Heaven, M.A. Hollas, Molecules 19, 16998–17025 (2014)
N.K. Kim, A. Murali, V. DeRose, Chem. Biol. 11, 939–948 (2004)
G.P.G. Grant, P.Z. Qin, Nucleic Acids Res. 35, 1–8 (2007)
S. Delannoy, I.L. Urbatsch, G. Tombline, A.E. Senior, P.D. Vogel, Biochemistry 44, 14010–14019 (2005)
M. Haller, U. Hoffmann, T. Schanding, R.S. Goody, P.D. Vogel, J. Biol. Chem. 272, 30103–30107 (1997)
J. Bhatnagar, P.P. Borbat, A.M. Pollard, A.M. Bilwes, J.H. Freed, B.R. Crane, Biochemistry 49, 3824–3841 (2010)
A.K. Eaton, R.C. Stewart, Biochemistry 48, 6412–6422 (2010)
A.M. Bilwes, C.M. Quezada, L.R. Croal, B.R. Crane, M.I. Simon, Nat. Struct. Biol. 8, 353–360 (2001)
L. Lauinger, A. Diernfellner, S. Falk, M. Brunner, Nat. Commun. 5, 3598 (2014)
K.S. Conrad, J.M. Hurley, J. Widom, C.S. Ringelburg, J.J. Loros, J.C. Dunlap, B.R. Crane. EMBO J. 35, 1707–1719 (2016)
G.E. Bronnikov, S.D. Zakharov, Anal. Biochem. 131, 69–74 (1983)
P.D. Adams, P.V. Afonine, R.W. Grosse-Kunstleve, R.J. Read, J.S. Richardson, D.C. Richardson, T.C. Terwilliger, Curr. Opin. Struct. Biol. 19, 566–572 (2009). https://doi.org/10.1016/j.sbi.2009.07.014
P. Emsley, B. Lohkamp, W.G. Scott, K. Cowtan, Acta Crystallogr. Sect. D Biol. Crystallogr. 66, 486–501 (2010)
P.P. Borbat, R.H. Crepeau, J.H. Freed, J. Magn. Reson. 167, 155–167 (1997)
P.P. Borbat, H.S. Mchaourab, J.H. Freed, J. Am. Chem. Soc. 124, 5304–5314 (2002)
M. Srivastava, C.L. Anderson, J.H. Freed, IEEE Access. 4, 3862–3877 (2016)
M. Srivastava, J.H. Freed, J. Phys. Chem. Lett. 8, 5648–5655 (2017)
Acknowledgements
This work supported by a National Science Foundation (NSF) Graduate Research Fellowship to ARM (2014155468) and a National Institutes of Health grant to BRC (R35GM122535). We are grateful to the National Biomedical Center for Advanced ESR Technology supported through NIH grant P41GM103521 to J.R. Freed and NE-CAT of the Advanced Photon Source at Argonne National Labs (NIH Grant P41GM103403) for access to X-ray data collection facilities. Coordinates were deposited in the Protein Data Bank under ID 6MI6. We thank Siddharth Chandrasekaran and Daniyal Tariq for assistance with cw-ESR experiments, Madhur Srivastava for help with data processing and Peter Borbat for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muok, A.R., Chua, T.K., Le, H. et al. Nucleotide Spin Labeling for ESR Spectroscopy of ATP-Binding Proteins. Appl Magn Reson 49, 1385–1395 (2018). https://doi.org/10.1007/s00723-018-1070-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-018-1070-6