Abstract
Summary
By integrating the multilevel biological evidence and bioinformatics analyses, the present study represents a systemic endeavor to identify BMD-associated genes and their roles in skeletal metabolism.
Introduction
Single-nucleotide polymorphism (SNP)-based genome-wide association studies (GWASs) have already identified about 100 loci associated with bone mineral density (BMD), but these loci only explain a small proportion of heritability to osteoporosis risk. In the present study, we performed a gene-based analysis of the largest GWASs in the bone field to identify additional BMD-associated genes.
Methods
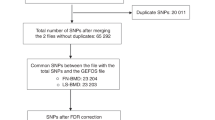
BMD-associated genes were identified by combining the summary statistic P values of SNPs across individual genes in the two consecutive meta-analyses of GWASs from the Genetic Factors for Osteoporosis (GEFOS) studies. The potential functionality of these genes to bone was partially assessed by differential gene expression analysis. Additionally, the consistency of the identification of potential bone mineral density (BMD)-associated variants were evaluated by estimating the correlation of the P values of the same single-nucleotide polymorphisms (SNPs)/genes between the two consecutive Genetic Factors for Osteoporosis Studies (GEFOS) with largely overlapping samples.
Results
Compared to the SNP-based analysis, the gene-based strategy identified additional BMD-associated genes with genome-wide significance and increased their mutual replication between the two GEFOS datasets. Among these BMD-associated genes, three novel genes (UBTF, AAAS, and C11orf58) were partially validated at the gene expression level. The correlation analysis presented a moderately high between-study consistency of potential BMD-associated variants.
Conclusions
Gene-based analysis as a supplementary strategy to SNP-based genome-wide association studies, when applied here, is shown that it helped identify some novel BMD-associated genes. In addition to its empirically increased statistical power, gene-based analysis also provides a higher testing stability for identification of BMD genes.



Similar content being viewed by others
Abbreviations
- AAAS:
-
Aladin WD repeat nucleoporin
- AOGC:
-
Australasian Osteoporosis Genetics Consortium
- BMD:
-
Bone mineral density
- CCNB1:
-
Cyclin B1
- DEGs:
-
Differentially expressed genes
- ERF:
-
Erasmus Rucphen Family Study
- FA:
-
Forearm
- FDR:
-
False discovery rate
- FHS:
-
Framingham Heart Study
- FN:
-
Femoral neck
- GATES:
-
Extended Simes procedure method
- GEFOS:
-
Genetic Factors for Osteoporosis Studies
- GEO:
-
Gene Expression Omnibus
- GO:
-
Gene ontology
- GWASs:
-
Genome-wide association studies
- HYST:
-
Hybrid set-based test
- KGG:
-
Knowledge-based mining system for genome-wide genetic studies
- LD:
-
Linkage disequilibrium
- LS:
-
Lumbar spine
- NHGRI:
-
National Human Genome Research Institute
- NUP155:
-
Nucleoporin 155
- PPI:
-
Protein-protein interaction
- QQ:
-
Quantile-quantile
- RANK:
-
Receptor activator of NFKB
- RANKL:
-
Receptor activator of NFKB ligand
- rRNA:
-
Ribosomal RNA
- RS-I:
-
Rotterdam Study-I
- SNP:
-
Single-nucleotide polymorphism
- TWINSUK:
-
TwinsUK
- UBTF:
-
Upstream binding transcription factor
- WHO:
-
World Health Organization
References
Kanis JA, Borgstrom F, de Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N (2005) Assessment of fracture risk. Osteoporos Int 16(6):581–589
Richards JB, Zheng HF, Spector TD (2012) Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet 13(8):576–588
Liu YJ, Zhang L, Papasian CJ, Deng HW (2014) Genome-wide association studies for osteoporosis: a 2013 update. J Bone Metab 21(2):99–116
Deng HW, Mahaney MC, Williams JT, Li J, Conway T, Davies KM, Li JL, Deng H, Recker RR (2002) Relevance of the genes for bone mass variation to susceptibility to osteoporotic fractures and its implications to gene search for complex human diseases. Genet Epidemiol 22(1):12–25
Evangelou E, Ioannidis JP (2013) Meta-analysis methods for genome-wide association studies and beyond. Nat Rev Genet 14(6):379–389
Pei YF, Zhang L, Papasian CJ, Wang YP, Deng HW (2014) On individual genome-wide association studies and their meta-analysis. Hum Genet 133(3):265–279
Ioannidis JP et al (2001) Replication validity of genetic association studies. Nat Genet 29(3):306–309
McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JPA, Hirschhorn JN (2008) Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet 9(5):356–369
Rivadeneira F et al (2009) Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41(11):1199–1206
Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OME, Amin N, Kemp JP, Koller DL, Li G, Liu CT, Minster RL, Moayyeri A, Vandenput L, Willner D, Xiao SM, Yerges-Armstrong LM, Zheng HF, Alonso N, Eriksson J, Kammerer CM, Kaptoge SK, Leo PJ, Thorleifsson G, Wilson SG, Wilson JF, Aalto V, Alen M, Aragaki AK, Aspelund T, Center JR, Dailiana Z, Duggan DJ, Garcia M, Garcia-Giralt N, Giroux S, Hallmans G, Hocking LJ, Husted LB, Jameson KA, Khusainova R, Kim GS, Kooperberg C, Koromila T, Kruk M, Laaksonen M, Lacroix AZ, Lee SH, Leung PC, Lewis JR, Masi L, Mencej-Bedrac S, Nguyen TV, Nogues X, Patel MS, Prezelj J, Rose LM, Scollen S, Siggeirsdottir K, Smith AV, Svensson O, Trompet S, Trummer O, van Schoor NM, Woo J, Zhu K, Balcells S, Brandi ML, Buckley BM, Cheng S, Christiansen C, Cooper C, Dedoussis G, Ford I, Frost M, Goltzman D, González-Macías J, Kähönen M, Karlsson M, Khusnutdinova E, Koh JM, Kollia P, Langdahl BL, Leslie WD, Lips P, Ljunggren Ö, Lorenc RS, Marc J, Mellström D, Obermayer-Pietsch B, Olmos JM, Pettersson-Kymmer U, Reid DM, Riancho JA, Ridker PM, Rousseau F, lagboom PES, Tang NLS, Urreizti R, van Hul W, Viikari J, Zarrabeitia MT, Aulchenko YS, Castano-Betancourt M, Grundberg E, Herrera L, Ingvarsson T, Johannsdottir H, Kwan T, Li R, Luben R, Medina-Gómez C, Th Palsson S, Reppe S, Rotter JI, Sigurdsson G, van Meurs JBJ, Verlaan D, Williams FMK, Wood AR, Zhou Y, Gautvik KM, Pastinen T, Raychaudhuri S, Cauley JA, Chasman DI, Clark GR, Cummings SR, Danoy P, Dennison EM, Eastell R, Eisman JA, Gudnason V, Hofman A, Jackson RD, Jones G, Jukema JW, Khaw KT, Lehtimäki T, Liu Y, Lorentzon M, McCloskey E, Mitchell BD, Nandakumar K, Nicholson GC, Oostra BA, Peacock M, Pols HAP, Prince RL, Raitakari O, Reid IR, Robbins J, Sambrook PN, Sham PC, Shuldiner AR, Tylavsky FA, van Duijn CM, Wareham NJ, Cupples LA, Econs MJ, Evans DM, Harris TB, Kung AWC, Psaty BM, Reeve J, Spector TD, Streeten EA, Zillikens MC, Thorsteinsdottir U, Ohlsson C, Karasik D, Richards JB, Brown MA, Stefansson K, Uitterlinden AG, Ralston SH, Ioannidis JPA, Kiel DP, Rivadeneira F (2012) Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 44(5):491–501
Zheng HF et al (2015) Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature 526(7571):112–117
Tak YG, Farnham PJ (2015) Making sense of GWAS: using epigenomics and genome engineering to understand the functional relevance of SNPs in non-coding regions of the human genome. Epigenetics Chromatin 8:57
Hazelett DJ, Rhie SK, Gaddis M, Yan C, Lakeland DL, Coetzee SG, Ellipse/GAME-ON consortium, Practical consortium, Henderson BE, Noushmehr H, Cozen W, Kote-Jarai Z, Eeles RA, Easton DF, Haiman CA, Lu W, Farnham PJ, Coetzee GA (2014) Comprehensive functional annotation of 77 prostate cancer risk loci. PLoS Genet 10(1):e1004102
Spain SL, Barrett JC (2015) Strategies for fine-mapping complex traits. Hum Mol Genet 24(R1):R111–R119
Hagg S et al (2015) Gene-based meta-analysis of genome-wide association studies implicates new loci involved in obesity. Hum Mol Genet 24(23):6849–6860
Wood AR, Hernandez DG, Nalls MA, Yaghootkar H, Gibbs JR, Harries LW, Chong S, Moore M, Weedon MN, Guralnik JM, Bandinelli S, Murray A, Ferrucci L, Singleton AB, Melzer D, Frayling TM (2011) Allelic heterogeneity and more detailed analyses of known loci explain additional phenotypic variation and reveal complex patterns of association. Hum Mol Genet 20(20):4082–4092
Li MX, Gui HS, Kwan JSH, Sham PC (2011) GATES: a rapid and powerful gene-based association test using extended Simes procedure. Am J Hum Genet 88(3):283–293
Wilk MB, Gnanadesikan R (1968) Probability plotting methods for the analysis of data. Biometrika 55(1):1–17
Li MX, Kwan JS, Sham PC (2012) HYST: a hybrid set-based test for genome-wide association studies, with application to protein-protein interaction-based association analysis. Am J Hum Genet 91(3):478–488
Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C (2015) STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43(Database issue):D447–D452
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10(1):101–129
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J (2009) ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25(8):1091–1093
Bindea G, Galon J, Mlecnik B (2013) CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics 29(5):661–663
Ramasamy A, Mondry A, Holmes CC, Altman DG (2008) Key issues in conducting a meta-analysis of gene expression microarray datasets. PLoS Med 5(9):e184
Chen XD, Xiao P, Lei SF, Liu YZ, Guo YF, Deng FY, Tan LJ, Zhu XZ, Chen FR, Recker RR, Deng HW (2010) Gene expression profiling in monocytes and SNP association suggest the importance of the STAT1 gene for osteoporosis in both Chinese and Caucasians. J Bone Miner Res 25(2):339–355
Lei SF, Wu S, Li LM, Deng FY, Xiao SM, Jiang C, Chen Y, Jiang H, Yang F, Tan LJ, Sun X, Zhu XZ, Liu MY, Liu YZ, Chen XD, Deng HW (2009) An in vivo genome wide gene expression study of circulating monocytes suggested GBP1, STAT1 and CXCL10 as novel risk genes for the differentiation of peak bone mass. Bone 44(5):1010–1014
Liu YZ, Dvornyk V, Lu Y, Shen H, Lappe JM, Recker RR, Deng HW (2005) A novel pathophysiological mechanism for osteoporosis suggested by an in vivo gene expression study of circulating monocytes. J Biol Chem 280(32):29011–29016
Reppe S, Refvem H, Gautvik VT, Olstad OK, Høvring PI, Reinholt FP, Holden M, Frigessi A, Jemtland R, Gautvik KM (2010) Eight genes are highly associated with BMD variation in postmenopausal Caucasian women. Bone 46(3):604–612
Xiao P, Chen Y, Jiang H, Liu YZ, Pan F, Yang TL, Tang ZH, Larsen JA, Lappe JM, Recker RR, Deng HW (2008) In vivo genome-wide expression study on human circulating B cells suggests a novel ESR1 and MAPK3 network for postmenopausal osteoporosis. J Bone Miner Res 23(5):644–654
(2003) Prevention and management of osteoporosis. World Health Organ Tech Rep Ser 921: p. 1–164, back cover
Zhou PR, Xu XJ, Zhang ZL, Liao EY, Chen DC, Liu J, Wu W, Jiang Y, Wang O, Xia WB, Xing XP, Xu L, Li M (2015) SOST polymorphisms and response to alendronate treatment in postmenopausal Chinese women with osteoporosis. Pharmacogenomics 16(10):1077–1088
Dallas SL, Prideaux M, Bonewald LF (2013) The osteocyte: an endocrine cell … and more. Endocr Rev 34(5):658–690
Horowitz MC, Fretz JA, Lorenzo JA (2010) How B cells influence bone biology in health and disease. Bone 47(3):472–479
He H, Cao S, Niu T, Zhou Y, Zhang L, Zeng Y, Zhu W, Wang YP, Deng HW (2016) Network-based meta-analyses of associations of multiple gene expression profiles with bone mineral density variations in women. PLoS One 11(1):e0147475
Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff L, Parkinson H (2014) The NHGRI GWAS catalog, a curated resource of SNP-trait associations. Nucleic Acids Res 42(Database issue):D1001–D1006
Koller DL, Ichikawa S, Johnson ML, Lai D, Xuei X, Edenberg HJ, Conneally PM, Hui SL, Johnston CC, Peacock M, Foroud T, Econs MJ (2005) Contribution of the LRP5 gene to normal variation in peak BMD in women. J Bone Miner Res 20(1):75–80
Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO (2004) Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res 19(12):2033–2040
Styrkarsdottir U, Thorleifsson G, Gudjonsson SA, Sigurdsson A, Center JR, Lee SH, Nguyen TV, Kwok TCY, Lee JSW, Ho SC, Woo J, Leung PC, Kim BJ, Rafnar T, Kiemeney LA, Ingvarsson T, Koh JM, Tang NLS, Eisman JA, Christiansen C, Sigurdsson G, Thorsteinsdottir U, Stefansson K (2016) Sequence variants in the PTCH1 gene associate with spine bone mineral density and osteoporotic fractures. Nat Commun 7:10129
Sanij E, Diesch J, Lesmana A, Poortinga G, Hein N, Lidgerwood G, Cameron DP, Ellul J, Goodall GJ, Wong LH, Dhillon AS, Hamdane N, Rothblum LI, Pearson RB, Haviv I, Moss T, Hannan RD (2015) A novel role for the pol I transcription factor UBTF in maintaining genome stability through the regulation of highly transcribed pol II genes. Genome Res 25(2):201–212
Ali SA, Dobson JR, Lian JB, Stein JL, van Wijnen AJ, Zaidi SK, Stein GS (2012) A RUNX2-HDAC1 co-repressor complex regulates rRNA gene expression by modulating UBF acetylation. J Cell Sci 125(Pt 11):2732–2739
Dumic M, Putarek NR, Kusec V, Barisic N, Koehler K, Huebner A (2016) Low bone mineral density for age/osteoporosis in triple A syndrome—an overlooked symptom of unexplained etiology. Osteoporos Int 27(2):521–526
Dauer WT, Worman HJ (2009) The nuclear envelope as a signaling node in development and disease. Dev Cell 17(5):626–638
Tan L, Liu R, Lei SF, Pan R, Yang TL, Yan H, Pei YF, Yang F, Zhang F, Pan F, Zhang YP, Hu HG, Levy S, Deng HW (2010) A genome-wide association analysis implicates SOX6 as a candidate gene for wrist bone mass. Sci China Life Sci 53(9):1065–1072
Acknowledgments
Full author lists of the two consortia (GEFOS2 and GEFOS-seq) were available in the Supplementary Acknowledgment.
Funding
This study was partially supported by and/or benefited from grants from National Institutes of Health [AR069055, U19 AG055373, R01 MH104680, R01AR059781 and P20GM109036], and Edward G. Schlieder Endowment to Tulane University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Zhu, W., Xu, C., Zhang, JG. et al. Gene-based GWAS analysis for consecutive studies of GEFOS. Osteoporos Int 29, 2645–2658 (2018). https://doi.org/10.1007/s00198-018-4654-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4654-y