Abstract
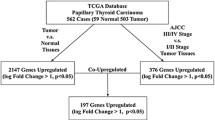
Glucagon is a crucial hormone involved in the maintenance of glucose homeostasis. Large efforts to define the role of glucagon receptor (GCGR) have been continuously made in recent years, but it is still incomplete about its function and mechanism. We performed this study to verify its potential impacts on papillary thyroid carcinoma (PTC) progression. Correlation between GCGR expression and PTC was elaborated using The Cancer Genome Atlas (TCGA) database. The Kaplan–Meier method was used to analyze the connection between GCGR expression and prognosis of PTC patients. GCGR expression was measured by quantitative real-time polymerase chain reaction (qRT-PCR) and western blot analysis; simultaneously, cell viability was elucidated using cell proliferation and colony formation assays following siRNAs strategy. Transwell analyses were conducted to measure the invasion and migration of PTC cells. Flow cytometry analysis was conducted to examine apoptotic ability. The cAMP ELISA kit was employed to measure the cAMP level in PTC cells. Our data determined that the expression level of GCGR was increased in PTC tissues and cells in contrast to normal tissues and Nthy-ori 3-1, respectively. Up-regulated GCGR expression was linked with the lower survival rate in patients with PTC. Functional analysis in vitro suggested that GCGR knockdown attenuated PTC cell proliferation, colony formation, invasion, and migration whilst intensified apoptosis. Down-regulated GCGR was able to increase cAMP level. Furthermore, reduction of GCGR could result in the inactivation of epithelial–mesenchymal transition (EMT) and P38/ERK pathways. In conclusion, the findings of this study disclosed that GCGR promoted PTC cell behaviors by mediating the EMT and P38/ERK pathways, serving as a potential diagnostic and prognostic biomarker as well as therapeutic target for PTC.





Similar content being viewed by others
References
Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (London, England). 2016;388(10061):2783–95. https://doi.org/10.1016/s0140-6736(16)30172-6.
Gezer E, Selek A, Tarkun I, Canturk Z, Cetinarslan B. Papillary thyroid carcinoma presenting as a primary renal tumor with multiple pulmonary and bone metastases: a case report. J Med Case Rep. 2019;13(1):95. https://doi.org/10.1186/s13256-019-2025-8.
Zhu J, Zhang Q, Jin XY, Cai JB, Chen X, Shi WB, et al. MiR-506 suppresses papillary thyroid carcinoma cell proliferation and metastasis via targeting IL17RD. Eur Rev Med Pharmacol Sci. 2019;23(7):2856–62. https://doi.org/10.26355/eurrev_201904_17563.
Du L, Wang Y, Sun X, Li H, Geng X, Ge M, et al. Thyroid cancer: trends in incidence, mortality and clinical-pathological patterns in Zhejiang Province, Southeast China. BMC Cancer. 2018;18(1):291. https://doi.org/10.1186/s12885-018-4081-7.
Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26(8):879–85. https://doi.org/10.1007/s00268-002-6612-1.
Wei ZL, Gao AB, Wang Q, Lou XE, Zhao J, Lu QJ. MicroRNA-221 promotes papillary thyroid carcinoma cell migration and invasion via targeting RECK and regulating epithelial-mesenchymal transition. Oncotargets Ther. 2019;12:2323–33. https://doi.org/10.2147/OTT.S190364.
Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55(1):167–94. https://doi.org/10.1124/pr.55.1.6.
Bansal P, Wang Q. Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab. 2008;295(4):E751–61. https://doi.org/10.1152/ajpendo.90295.2008.
Kobilka BK. G protein coupled receptor structure and activation. Biochem Biophys Acta. 2007;1768(4):794–807. https://doi.org/10.1016/j.bbamem.2006.10.021.
Wang F, Dai CQ, Zhang LR, Bing C, Qin J, Liu YF. Downregulation of Lgr6 inhibits proliferation and invasion and increases apoptosis in human colorectal cancer. Int J Mol Med. 2018;42(1):625–32. https://doi.org/10.3892/ijmm.2018.3633.
Ye R, Pi M, Cox JV, Nishimoto SK, Quarles LD. CRISPR/Cas9 targeting of GPRC6A suppresses prostate cancer tumorigenesis in a human xenograft model. J Exp Clin Cancer Res CR. 2017;36(1):90. https://doi.org/10.1186/s13046-017-0561-x.
Demenais F, Mohamdi H, Chaudru V, Goldstein AM, Newton Bishop JA, Bishop DT, et al. Association of MC1R variants and host phenotypes with melanoma risk in CDKN2A mutation carriers: a GenoMEL study. J Natl Cancer Inst. 2010;102(20):1568–83. https://doi.org/10.1093/jnci/djq363.
Shang D, Li Z, Zhu Z, Chen H, Zhao L, Wang X, et al. Baicalein suppresses 17-beta-estradiol-induced migration, adhesion and invasion of breast cancer cells via the G protein-coupled receptor 30 signaling pathway. Oncol Rep. 2015;33(4):2077–85. https://doi.org/10.3892/or.2015.3786.
Wei W, Chen ZJ, Zhang KS, Yang XL, Wu YM, Chen XH, et al. The activation of G protein-coupled receptor 30 (GPR30) inhibits proliferation of estrogen receptor-negative breast cancer cells in vitro and in vivo. Cell Death Dis. 2014;5:e1428. https://doi.org/10.1038/cddis.2014.398.
Kanda R, Hiraike H, Wada-Hiraike O, Ichinose T, Nagasaka K, Sasajima Y, et al. Expression of the glucagon-like peptide-1 receptor and its role in regulating autophagy in endometrial cancer. BMC Cancer. 2018;18(1):657. https://doi.org/10.1186/s12885-018-4570-8.
Wang W, Li Y, Zhu JY, Fang D, Ding HF, Dong Z, et al. Triple negative breast cancer development can be selectively suppressed by sustaining an elevated level of cellular cyclic AMP through simultaneously blocking its efflux and decomposition. Oncotarget. 2016;7(52):87232–45. https://doi.org/10.18632/oncotarget.13601.
Zhang DD, Li Y, Xu Y, Kim J, Huang S. Phosphodiesterase 7B/microRNA-200c relationship regulates triple-negative breast cancer cell growth. Oncogene. 2019;38(7):1106–20. https://doi.org/10.1038/s41388-018-0499-2.
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25(5):666–81. https://doi.org/10.1016/j.ccr.2014.03.010.
Du Z, Wang Q, Ma G, Jiao J, Jiang D, Zheng X, et al. Inhibition of Nrf2 promotes the antitumor effect of Pinelliae rhizome in papillary thyroid cancer. J Cell Physiol. 2019;234(8):13867–77. https://doi.org/10.1002/jcp.28069.
Wang N, Li Y, Wei J, Pu J, Liu R, Yang Q, et al. TBX1 functions as a tumor suppressor in thyroid cancer through inhibiting the activities of the PI3K/AKT and MAPK/ERK pathways. Thyroid. 2019;29(3):378–94. https://doi.org/10.1089/thy.2018.0312.
Ferrari SM, Antonelli A, Guidi P, Bernardeschi M, Scarcelli V, Fallahi P, et al. Genotoxicity evaluation of the soybean isoflavone genistein in human papillary thyroid cancer cells. Study of its potential use in thyroid cancer therapy. Nut Cancer. 2019. https://doi.org/10.1080/01635581.2019.1604004.
Okamoto H, Cavino K, Na E, Krumm E, Kim SY, Cheng X, et al. Glucagon receptor inhibition normalizes blood glucose in severe insulin-resistant mice. Proc Natl Acad Sci USA. 2017;114(10):2753–8. https://doi.org/10.1073/pnas.1621069114.
Ben-Zvi D, Barrandon O, Hadley S, Blum B, Peterson QP, Melton DA. Angptl4 links alpha-cell proliferation following glucagon receptor inhibition with adipose tissue triglyceride metabolism. Proc Natl Acad Sci USA. 2015;112(50):15498–503. https://doi.org/10.1073/pnas.1513872112.
Sipos B, Sperveslage J, Anlauf M, Hoffmeister M, Henopp T, Buch S, et al. Glucagon cell hyperplasia and neoplasia with and without glucagon receptor mutations. J Clin Endocrinol Metab. 2015;100(5):E783–8. https://doi.org/10.1210/jc.2014-4405.
Graham GV, Conlon JM, Abdel-Wahab YH, Flatt PR. Glucagon-related peptides from phylogenetically ancient fish reveal new approaches to the development of dual GCGR and GLP1R agonists for type 2 diabetes therapy. Peptides. 2018;110:19–29. https://doi.org/10.1016/j.peptides.2018.10.013.
Wang MY, Yan H, Shi Z, Evans MR, Yu X, Lee Y, et al. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc Natl Acad Sci USA. 2015;112(8):2503–8. https://doi.org/10.1073/pnas.1424934112.
Luo J, Phillips L, Liu S, Wactawski-Wende J, Margolis KL. Diabetes, diabetes treatment, and risk of thyroid cancer. J Clin Endocrinol Metab. 2016;101(3):1243–8. https://doi.org/10.1210/jc.2015-3901.
Perros P, McCrimmon RJ, Shaw G, Frier BM. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diab Med J Br Diab Assoc. 1995;12(7):622–7.
Celani MF, Bonati ME, Stucci N. Prevalence of abnormal thyrotropin concentrations measured by a sensitive assay in patients with type 2 diabetes mellitus. Diabetes research (Edinburgh, Scotland). 1994;27(1):15–25.
Kim T, Nason S, Holleman C, Pepin M, Wilson L, Berryhill TF, et al. Glucagon receptor signaling regulates energy metabolism via hepatic farnesoid X receptor and fibroblast growth factor. Diabetes. 2018;67(9):1773–82. https://doi.org/10.2337/db17-1502.
Ali S, Ussher JR, Baggio LL, Kabir MG, Charron MJ, Ilkayeva O, et al. Cardiomyocyte glucagon receptor signaling modulates outcomes in mice with experimental myocardial infarction. Mol Metab. 2015;4(2):132–43. https://doi.org/10.1016/j.molmet.2014.11.005.
Karwi QG, Zhang L, Wagg CS, Wang W, Ghandi M, Thai D, et al. Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction. Cardiovasc Diabetol. 2019;18(1):1. https://doi.org/10.1186/s12933-019-0806-4.
Sinclair EM, Yusta B, Streutker C, Baggio LL, Koehler J, Charron MJ, et al. Glucagon receptor signaling is essential for control of murine hepatocyte survival. Gastroenterology. 2008;135(6):2096–106. https://doi.org/10.1053/j.gastro.2008.07.075.
Naviglio S, Di Gesto D, Illiano F, Chiosi E, Giordano A, Illiano G, et al. Leptin potentiates antiproliferative action of cAMP elevation via protein kinase A down-regulation in breast cancer cells. J Cell Physiol. 2010;225(3):801–9. https://doi.org/10.1002/jcp.22288.
Ye X, Brabletz T, Kang Y, Longmore GD, Nieto MA, Stanger BZ, et al. Upholding a role for EMT in breast cancer metastasis. Nature. 2017;547(7661):e1–3. https://doi.org/10.1038/nature22816.
Luo X, Qiu Y, Jiang Y, Chen F, Jiang L, Zhou Y, et al. Long non-coding RNA implicated in the invasion and metastasis of head and neck cancer: possible function and mechanisms. Mol Cancer. 2018;17(1):14. https://doi.org/10.1186/s12943-018-0763-7.
Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12(4):361–73. https://doi.org/10.1007/s11684-018-0656-6.
Cho JH, Hong WG, Jung YJ, Lee J, Lee E, Hwang SG, et al. Gamma-Ionizing radiation-induced activation of the EGFR-p38/ERK-STAT3/CREB-1-EMT pathway promotes the migration/invasion of non-small cell lung cancer cells and is inhibited by podophyllotoxin acetate. Tumour Biol J Int Soc Oncodev Biol Med. 2016;37(6):7315–25. https://doi.org/10.1007/s13277-015-4548-y.
Lei YY, Wang WJ, Mei JH, Wang CL. Mitogen-activated protein kinase signal transduction in solid tumors. Asian Pac J Cancer Prev APJCP. 2014;15(20):8539–48.
Zhang G, He J, Ye X, Zhu J, Hu X, Shen M, et al. beta-Thujaplicin induces autophagic cell death, apoptosis, and cell cycle arrest through ROS-mediated Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma. Cell Death Dis. 2019;10(4):255. https://doi.org/10.1038/s41419-019-1492-6.
Wang Y, Yu L, Wang T. MicroRNA-374b inhibits the tumor growth and promotes apoptosis in non-small cell lung cancer tissue through the p38/ERK signaling pathway by targeting JAM-2. J Thorac Dis. 2018;10(9):5489–98. https://doi.org/10.21037/jtd.2018.09.93.
Li GC, Cao XY, Li YN, Qiu YY, Li YN, Liu XJ, et al. MicroRNA-374b inhibits cervical cancer cell proliferation and induces apoptosis through the p38/ERK signaling pathway by binding to JAM-2. J Cell Physiol. 2018;233(9):7379–90. https://doi.org/10.1002/jcp.26574.
Tang G, Du R, Tang Z, Kuang Y. MiRNALet-7a mediates prostate cancer PC-3 cell invasion, migration by inducing epithelial-mesenchymal transition through CCR43/MAPK pathway. J Cell Biochem. 2018;119(4):3725–31. https://doi.org/10.1002/jcb.26595.
Song B, Li R, Zuo Z, Tan J, Liu L, Ding D, et al. LncRNA ENST00000539653 acts as an oncogenic factor via MAPK signalling in papillary thyroid cancer. BMC Cancer. 2019;19(1):297. https://doi.org/10.1186/s12885-019-5533-4.
Liu F, Yin R, Chen X, Chen W, Qian Y, Zhao Y, et al. Over-expression of miR-206 decreases the Euthyrox-resistance by targeting MAP4K3 in papillary thyroid carcinoma. Biomed Pharmacother. 2019;114:108605. https://doi.org/10.1016/j.biopha.2019.108605.
Fuziwara CS, Saito KC, Leoni SG, Waitzberg AFL, Kimura ET. The highly expressed FAM83F protein in papillary thyroid cancer exerts a pro-oncogenic role in thyroid follicular cells. Front Endocrinol. 2019;10:134. https://doi.org/10.3389/fendo.2019.00134.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, HC., Chen, XR., Sun, HF. et al. Tumor promoting effects of glucagon receptor: a promising biomarker of papillary thyroid carcinoma via regulating EMT and P38/ERK pathways. Human Cell 33, 175–184 (2020). https://doi.org/10.1007/s13577-019-00284-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-019-00284-y