Abstract
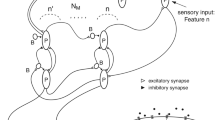
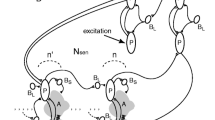
Interaction between sensory and motor cortices is crucial for perceptual decision-making, in which intracortical inhibition might have an important role. We simulated a neural network model consisting of a sensory network (NS) and a motor network (NM) to elucidate the significance of their interaction in perceptual decision-making in association with the level of GABA in extracellular space: extracellular GABA concentration. Extracellular GABA molecules acted on extrasynaptic receptors embedded in membranes of pyramidal cells and suppressed them. A reduction in extracellular GABA concentration either in NS or NM increased the rate of errors in perceptual decision-making, for which an increase in ongoing-spontaneous fluctuations in subthreshold neuronal activity in NM prior to sensory stimulation was responsible. Feedback (NM-to-NS) signaling enhanced selective neuronal responses in NS, which in turn increased stimulus-evoked neuronal activity in NM. We suggest that GABA in extracellular space contributes to reducing variability in motor cortex activity at a resting state and thereby the motor cortex can respond correctly to a subsequent sensory stimulus. Feedback signaling from the motor cortex improves the selective responsiveness of the sensory cortex, which ensures the fidelity of information transmission to the motor cortex, leading to reliable perceptual decision-making.







Similar content being viewed by others
References
Bianchim, M.T., Haas, K.F., Macdonald, R.L. (2001). Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAa receptors. J. Neurosci., 21, 1127–36.
Bianchim, M.T., Haas, K.F., Macdonald, R.L. (2002). Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the delta subunit. Neuropharmacology, 43, 492–502.
Brickley, S.G., Cull-Candy, S.G., Farrant, M. (1996). Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol., 497.3, 753–759.
Brown, N., Kerby, J., Bonnert, T.P., Whiting, P.J., Wafford, K.A. (2002). Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br. J. Pharmacol., 136, 965–974.
deCharms, R.C., & Zador, A. (2000). Neural representation and the cortical code. Annu. Rev. Neurosci., 23, 613–647.
Drasbek, K.R., & Jensen, K. (2006). THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb. Cortex, 16, 1134–1141.
Edden, R.A., Muthukumaraswamy, S.D., Freeman, T.C., Singh, K.D. (2009). Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci., 29, 15721–15726.
Farrant, M., & Nusser, Z. (2005). Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci., 6, 215–229.
Fox, M.D., Snyder, A.Z., Vincent, J.L., Raichle, M.E. (2007). Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron, 56, 171–184.
Gonzalez-Burgos, G., Fish, K.N., Lewis, D.A. (2011). GABA Neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plasticity, 2011, 723184.
Hasler, G., van der Veen, J.W., Tumonis, T., Meyers, N., Shen, J., Drevets, W.C. (2007). Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry, 64, 193–200.
Hatsopoulos, N.G., & Suminski, A.J. (2011). Sensing with the motor cortex. Neuron, 72, 477–487.
Hoshino, O. (2009). GABA Transporter preserving ongoing spontaneous neuronal activity at firing subthreshold. Neural Comput., 21, 1683–1713.
Hoshino, O. (2012). Regulation of ambient GABA levels by neuron-glia signaling for reliable perception of multisensory events. Neural Comput., 24, 2964–2993.
Hoshino, O. (2014). Balanced crossmodal excitation and inhibition essential for maximizing multisensory gain. Neural Comput., 26, 1362–1385.
Hoshino, O., Zheng, M., Watanabe, K. (2018). Perceptual judgments via sensory-motor interaction assisted by cortical GABA. J. Comput. Neurosci., 44, 233–251.
Jones, M.V., & Westbrook, G.L. (1995). Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron, 15, 181–91.
Kolasinski, J., Logan, J.P., Hinson, E.L., Manners, D., Divanbeighi, Z.A.P., Makin, T.R., Emir, U.E., Stagg, C.J. (2017). A mechanistic link from GABA to cortical architecture and perception. Current Biology, 27, 1685–1691.
Lerma, J., Herranz, A.S., Herreras, O., Abraira, V., Martin, D.R. (1986). In vivo determination of extracellular concentration of amino acids in the rat hippocampus: A method based on brain dialysis and computerized analysis. Brain Res., 384, 145–155.
Leventhal, A.G., Thompson, K.G., Liu, D., Zhou, Y., Ault, S.J. (1995). Concomitant sensitivity to orientation, direction, and color of cells in layers 2, 3, and 4 of monkey striate cortex. J. Neurosci., 15, 1808–1818.
Maconochie, D.J., Zempel, J.M., Steinbach, J.H. (1994). How quickly can GABAA receptors open?. Neuron, 12, 61–71.
Makino, H., Hwang, E.J., Hedrick, N.G., Komiyama, T. (2016). Circuit Mechanisms of Sensorimotor Learning. Neuron, 92, 705–721.
Manita, S., Suzuki, T., Homma, C., Matsumoto, T., Odagawa, M., Yamada, K., Ota, K., Matsubara, V., Inutsuka, A., Sato, M., Ohkura, M., Yamanaka, A., Yanagawa, Y., Nakai, J., Hayashi, Y., Larkum, M.E., Murayama, M. (2015). A Top-Down Cortical Circuit for Accurate Sensory Perception. Neuron, 86, 1304–1316.
Mao, T., Kusefoglu, D., Hooks, B.M., Huber, D., Petreanu, L., Svoboda, K. (2011). Long-range neuronal circuits underlying the interaction betweeN Ssory and motor cortex. Neuron, 72, 111–123.
Matyas, F., Sreenivasan, V., Marbach, F., Wacongne, C., Barsy, B., Mateo, C., Aronoff, R., Petersen, C.C. (2010). Motor control by sensory cortex. Science, 330, 1240–1243.
Mountcastle, V.B. (1997). The columnar organization of the neocortex. Brain: A Journal of Neurology, 120, 701–722.
Nusser, Z., Roberts, J.D., Baude, A., Richards, J.G., Somogyi, P. (1995). Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. The Journal of Neuroscience, 5, 2948–2960.
Ortinski, P.I., Turner, J.R., Barberis, A., Motamedi, G., Yasuda, R.P., Wolfe, B.B., Kellar, K.J., Vicini, S. (2006). Deletion of the GABA(a) receptor alpha1 subunit increases tonic GABA(a) receptor current: a role for GABA uptake transporters. The Journal of Neuroscience, 26, 9323–9331.
Puts, N.A., Edden, R.A., Evans, C.J., McGlone, F., McGonigle, D.J. (2011). Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J. Neurosci., 31, 16556–16560.
Sachidhanandam, S., Sreenivasan, V., Kyriakatos, A., Kremer, Y., Petersen, C.C. (2013). Membrane potential correlates of sensory perception in mouse barrel cortex. Nature Neuroscience, 16, 1671–1677.
Sandberg, K., Blicher, J.U., Dong, M.Y., Rees, G., Near, J., Kanai, R. (2014). Occipital GABA correlates with cognitive failures in daily life. Neuroimage, 87, 55–60.
Saxena, N.C., & Macdonald, R.L. (1996). Properties of putative cerebellar gamma-aminobutyric acidA receptor isoforms. Mol. Pharmacol., 49, 567–579.
Schmolesky, M.T., Wang, Y., Pu, M., Leventhal, A.G. (2000). Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat. Neurosci., 3, 384–390.
Scimemi, A., Semyanov, A., Sperk, G., Kullmann, D.M., Walker, M.C. (2005). Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J. Neurosci., 25, 10016–10024.
Scimemi, A., Andersson, A., Heeroma, J.H., Strandberg, J., Rydenhag, B., McEvoy, A.W., Thom, M., Asztely, F., Walker. M.C. (2006). Tonic GABA(A) receptor-mediated currents in human brain. Eur. J. Neurosci., 24, 1157–1160.
Semyanov, A., Walker, M.C., Kullmann, D.M., Silver, R.A. (2004). Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci., 27, 262–269.
Siddoway, B., Hou, H., Xia, H. (2014). Molecular mechanisms of homeostatic synaptic downscaling. Neuropharmacology, 78, 38–44.
Soltesz, I., & Nusser, Z. (2001). Neurobiology. Background inhibition to the fore. Nature, 409, 24–25.
Somogyi, P., Takagi, H., Richards, J.G., Mohler, H. (1989). Subcellular localization of benzodiazepine/GABAA receptors in the cerebellum of rat, cat, and monkey using monoclonal antibodies. J. Neurosci., 9, 2197–2209.
Stagg, C.J., Bestmann, S., Constantinescu, A.O., Moreno, L.M., Allman, C., Mekle, R., Woolrich, M., Near, J., Johan-Berg, H., Rothwell, J.C. (2011). Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol., 589, 5845–5855.
Sumner, P., Edden, R.A., Bompas, A., Evans, C.J., Singh, K.D. (2010). More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat. Neurosci., 13, 825–827.
Tossman, U., Jonsson, G., Ungerstedt, U. (1986). Regional distribution and extracellular levels of amino acids in rat central nervous system. Acta Physiol. Scand., 127, 533–545.
Turrigiano, G.G., & Nelson, S.B. (2000). Hebb and homeostasis in neuronal plasticity. Curr. Opin. Neurobiol., 10, 358–364.
Xu, X., Ichida, J., Shostak, Y., Bonds, A.B., Casagrande, V.A. (2002). Are primate lateral geniculate nucleus (LGN) cells really sensitive to orientation or direction?. Vis. Neurosci., 19, 97–108.
Zach, N., Inbar, D., Grinvald, Y., Bergman, H., Vaadia, E. (2008). Emergence of novel representations in primary motor cortex and premotor neurons during associative learning. J. Neurosci., 28, 9545–9556.
Zagha, E., Casale, A.E., Sachdev, R.N., McGinley, M.J., McCormick, D.A. (2013). Motor cortex feedback influences sensory processing by modulating network state. Neuron, 79, 567–578.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Action Editor: Maxim Bazhenov
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hoshino, O., Kameno, R. & Watanabe, K. Reducing variability in motor cortex activity at a resting state by extracellular GABA for reliable perceptual decision-making. J Comput Neurosci 47, 191–204 (2019). https://doi.org/10.1007/s10827-019-00732-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-019-00732-6