Abstract
Summary
We assessed whether a bone resorption marker, measured early in the menopause transition (MT), is associated with change in femoral neck size and strength during the MT. Higher levels of bone resorption were associated with slower increases in femoral neck size and faster decreases in femoral neck strength.
Purpose
Composite indices of the femoral neck’s ability to withstand compressive (compression strength index, CSI) and impact (impact strength index, ISI) forces integrate DXA-derived femoral neck width (FNW), bone mineral density (BMD), and body size. During the menopause transition (MT), FNW increases, and CSI and ISI decrease. This proof-of-concept study assessed whether a bone resorption marker, measured early in the MT, is associated with rates of change in FNW, CSI and ISI during the MT.
Methods
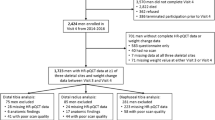
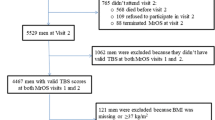
We used previously collected bone resorption marker (urine collagen type I N-telopeptide [U-NTX]) and femoral neck strength data from 696 participants from the Study of Women’s Health Across the Nation (SWAN), a longitudinal study of the MT in a multi-ethnic cohort of community-dwelling women.
Results
Adjusted for MT stage (pre- vs. early perimenopause), age, body mass index (BMI), bone resorption marker collection time, and study site in multivariable linear regression, bone resorption in pre- and early perimenopause was not associated with transmenopausal decline rate in femoral neck BMD. However, each standard deviation (SD) increase in bone resorption level was associated with 0.2% per year slower increase in FNW (p = 0.03), and 0.3% per year faster declines in CSI (p = 0.02) and ISI (p = 0.01). When restricted to women in early perimenopause, the associations of bone resorption with change in FNW, CSI, and ISI were similar to those in the full sample.
Conclusions
Measuring a bone resorption marker in pre- and early perimenopause may identify women who will experience the greatest loss in bone strength during the MT.

Similar content being viewed by others
References
Camacho P, Petak S, Binkley N, Clarke B, Harris S, Hurley D, Kleerekoper M, Lewiecki E, Miller P, Narula H, Pessah-Pollack R, Tangpricha V, Wimalawansa S, Watts N (2016) American association of clinical endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016--executive summary. Endocr Pract 22(9):1111–1118
Ishii S, Cauley JA, Greendale GA, Crandall CJ, Huang MH, Danielson ME, Karlamangla AS (2013) Trajectories of femoral neck strength in relation to the final menstrual period in a multi-ethnic cohort. Osteoporos Int 24(9):2471–2481
Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas P (2006) Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res 21(12):1856–1863. https://doi.org/10.1359/jbmr.060904
Riggs B, Melton ILR, Robb R, Camp J, Atkinson E, Peterson J, Rouleau P, McCollough C, Bouxsein M, Khosla S (2004) Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 19(12):1945–1954. https://doi.org/10.1359/JBMR.040916
Balena R, Shih M, Parfitt A (1992) Bone resorption and formation on the periosteal envelope of the ilium: a histomorphometric study in healthy women. J Bone Miner Res 7(12):1475–1482. https://doi.org/10.1002/jbmr.5650071216
Seeman E (2003) Periosteal bone formation--a neglected determinant of bone strength. N Engl J Med 349(4):320–323
Ahlborg H, Johnell O, Turner C, Rannevik G, Karlsson M (2003) Bone loss and bone size after menopause. N Engl J Med 349(4):327–334. https://doi.org/10.1056/NEJMoa022464
Leslie W, Lix L, Majumdar S, Morin S, Johansson H, Odén A, McCloskey E, Kanis J (2017) Total hip bone area affects fracture prediction with FRAX® in Canadian white women. J Clin Endocrinol Metab 102(11):4242–4249. https://doi.org/10.1210/jc.2017-01327
Karlamangla AS, Barrett-Connor E, Young J, Greendale GA (2004) Hip fracture risk assessment using composite indices of femoral neck strength: the Rancho Bernardo study. Osteoporos Int 15(1):62–70
Ishii S, Greendale GA, Cauley JA, Crandall CJ, Huang M-H, Danielson ME, Karlamangla AS (2012) Fracture risk assessment without race/ethnicity information. The Journal of Clinical Endocrinology & Metabolism 97(10):3593–3602
Danielson M, Beck T, Karlamangla A, Greendale G, Atkinson E, Lian Y, Khaled A, Keaveny T, Kopperdahl D, Ruppert K, Greenspan S, Vuga M, Cauley J (2013) A comparison of DXA and CT based methods for estimating the strength of the femoral neck in post-menopausal women. Osteoporos Int 24(4):1379–1388. https://doi.org/10.1007/s00198-012-2066-y
Greendale G, Sowers M, Han W, Huang M, Finkelstein J, Crandall C, Lee J, Karlamangla A (2012) Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: Results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res 27(1):111–118. https://doi.org/10.1002/jbmr.534
Shieh A, Ishii S, Greendale G, Cauley J, Lo J, Karlamangla A (2016) Urinary N-telopeptide and rate of bone loss over the menopause transition and early postmenopause. J Bone Miner Res 31:2057–2064. https://doi.org/10.1002/jbmr.2889
Sowers M, Zheng H, Greendale G, Neer R, Cauley J, Ellis J, Johnson S, Finkelstein J (2013) Changes in bone resorption across the menopause transition: effects of reproductive hormones, body size, and ethnicity. J Clin Endocrinol Metab 98(7):2854–2863. https://doi.org/10.1210/jc.2012-4113
Cauley JA, Danielson ME, Greendale GA, Finkelstein JS, Chang Y-F, Lo JC, Crandall CJ, Neer RM, Ruppert K, Meyn L, Prairie BA, Sowers MR (2012) Bone resorption and fracture across the menopausal transition: the Study of Women’s Health Across the Nation. Menopause 19(11):1200–1207
Shieh A, Han W, Ishii S, Greendale G, Crandall C, Karlamangla A (2016) Quantifying the balance between total bone formation and total bone resorption: an index of net bone formation. J Clin Endocrinol Metab 101(7):2802–2809. https://doi.org/10.1210/jc.2015-4262
Vasikaran SCC, Eastell R, Griesmacher A, Morris HA, Trenti T, Kanis JA (2011) International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med 49(8):1271–1274
Dresner-Pollak R, Parker R, Poku M, Thompson J, Seibel M, Greenspan S (1996) Biochemical markers of bone turnover reflect femoral bone loss in elderly women. Calcif Tissue Int 59(5):328–333
Garnero P, Sornay-Rendu E, Chapuy M, Delmas P (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11(3):337–349
Garnero P, Hausherr E, Chapuy M, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier P, Delmas P (1996) Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11(10):1531–1538
Ebeling P, Atley L, Guthrie J, Burger H, Dennerstein L, Hopper J, Wark J (1996) Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab 81(9):3366–3371. https://doi.org/10.1210/jcem.81.9.8784098
Rogers A, Hannon R, Eastell R (2000) Biochemical markers as predictors of rates of bone loss after menopause. J Bone Miner Res 15(7):1398–1404
Štěpán J, Pospichal J, Presl J, Pacovský V (1987) Bone loss and biochemical indices of bone remodeling in surgically induced postmenopausal women. Bone 8(5):279–284
Garnero P, Sornay-Rendu E, Duboeuf F, Delmas P (1999) Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res 14(9):1614–1621
Löfman O, Magnusson P, Toss G, Larsson L (2005) Common biochemical markers of bone turnover predict future bone loss: a 5-year follow-up study. Clin Chim Acta 356(1):67–75
Bauer D, Sklarin P, Stone K, Black D, Nevitt M, Ensrud K, Cummings S (1999) Biochemical markers of bone turnover and prediction of hip bone loss in older women: the study of osteoporotic fractures. J Bone Miner Res 14(8):1404–1410
Marques E, Gudnason V, Lang T, Sigurdsson G, Sigurdsson S, Aspelund T, Siggeirsdottir K, Launer L, Eiriksdottir G, Harris T (2016) Association of bone turnover markers with volumetric bone loss, periosteal apposition, and fracture risk in older men and women: the AGES-Reykjavik longitudinal study. Osteoporos Int 27(12):3485–3494. https://doi.org/10.1007/s00198-016-3675-7
Marques E, Gudnason V, Sigurdsson G, Lang T, Johannesdottir F, Siggeirsdottir K, Launer L, Eiriksdottir G, Harris T (2016) Are bone turnover markers associated with volumetric bone density, size, and strength in older men and women? The AGES-Reykjavik study. Osteoporos Int 27(5):1765–1776. https://doi.org/10.1007/s00198-015-3442-1
Orwoll ES (2003) Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res 18(6):949–954. https://doi.org/10.1359/jbmr.2003.18.6.949
Epker BN, Frost HM (1965) A histological study of remodeling at the periosteal, haversian canal, cortical endosteal, and trabecular endosteal surfaces in human rib. Anat Rec 152(2):129–135
Orwoll E, Orwoll S, Kohama S, Evans G, Turner R (2002) Periosteal bone formation and resorption are both active at the femoral neck: mechanisms for change in bone size. J Bone Miner Res 17(S1):S300
Frost HM (1982) Mechanical determinants of bone modeling. Metab Bone Dis Relat Res 4(4):217–229
Nickolas T, Leonard M, Shane E (2008) Chronic kidney disease and bone fracture: a growing concern. Kidney Int 74(6):721–731. https://doi.org/10.1038/ki.2008.264
Greendale G, Ishii S, Huang M, Karlamangla A (2013) Predicting the timeline to the final menstrual period: the study of women's health across the nation. J Clin Endocrinol Metab 98(4):1483–1491. https://doi.org/10.1210/jc.2012-3732
Vasikaran S, Eastell R, Bruyère O, Foldes A, Garnero P, Griesmacher A, McClung M, Morris H, Silverman S, Trenti T, Wahl D, Cooper C, Kanis J (2011) Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22(2):391–420. https://doi.org/10.1007/s00198-010-1501-1
Engelke K, Lang T, Khosla S, Qin L, Zysset P, Leslie WD, Shepherd JA, Shousboe JT (2015) Clinical use of quantitative computed tomography-based advanced techniques in the management of osteoporosis in adults: the 2015 ISCD official positions-part III. J Clin Densitom 18(3):393–407. https://doi.org/10.1016/j.jocd.2015.06.010
Nishiyama KK, Shane E (2013) Clinical imaging of bone microarchitecture with HR-pQCT. Curr Osteoporos Rep 11(2):147–155. https://doi.org/10.1007/s11914-013-0142-7
Zysset PK, Dall'ara E, Varga P, Pahr DH (2013) Finite element analysis for prediction of bone strength. BoneKEy reports 2:386. https://doi.org/10.1038/bonekey.2013.120
Acknowledgements
We thank the study staff at each site and all the women who participated in SWAN.
Funding
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). Additional support for this project was provided by NIA through P30-AG028748; UCLA Claude Pepper Older Adults Independence Center (PI: Reuben) funded by the National Institute of Aging (5P30AG028748); NIH/NCATS UCLA CTSI Grant Number UL1TR000124; and NIH Grant Number 5RO1AG033067 (PI: Karlamangla and Crandall). Dr. Shieh was supported by the UCLA Specialty Training and Advanced Research Program and the Iris Cantor-UCLA Women’s Health Center Executive Advisory Board. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All participants provided written informed consent, and each site obtained institutional review board approval.
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical Centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser–Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD—Chhanda Dutta 2016–present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
Rights and permissions
About this article
Cite this article
Shieh, A., Ishii, S., Greendale, G. et al. A bone resorption marker as predictor of rate of change in femoral neck size and strength during the menopause transition. Osteoporos Int 30, 2449–2457 (2019). https://doi.org/10.1007/s00198-019-05099-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-019-05099-z