Abstract
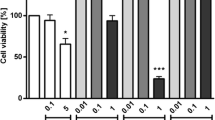
Several species of microalgae have been known to produce exopolysaccharides (EPS) with potential immune activity. In the present investigation, ethyl acetate fraction of crude EPS secreted by Dunaliella salina was explored for immunomodulatory activity against peripheral blood mononuclear cells (PBMC) and RAW 264.7 macrophages. Effect of EPS on cell growth and cytokines production were measured using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and ELISA respectively. Griess reagent was used for measuring the nitric oxide production in RAW 264.7 macrophages. FTIR analysis and mass spectroscopy were carried out for the characterization. Ethyl acetate fraction exhibited dose dependent increase in proliferative index and cytokines production (IFN-γ, TNF-α, TGF-β). At low concentration (250 and 500 µg/mL), it showed growth inhibition and at higher concentration (1000 and 1500 µg/mL), it enhanced the cell growth. Interestingly, the pronounced increased TNF-α production was observed in ethyl acetate fraction treated PBMC cells at higher concentration (750 and 1000 µg/mL) indicating the immunostimulatory effect. In RAW cells, concentration dependent diminished cell growth (IC50 = 691 µg/mL) and nitric oxide production (IC50 = 630 µg/mL) was observed. FTIR analysis showed the presence of polysaccharides due to the detection of hydroxyl (–OH), Carbonyl (C–O) and alkyl (C–H) groups. Mass spectroscopy results revealed ethyl acetate fraction as penta-saccharide (m/z = 887.56 and 886.54) which are confirmed to be hetero-polysaccharides consisting of hexoses and pentoses along with association of ions. These results suggest that penta-saccharide (ethyl acetate fraction) isolated from D. salina may have the potential to be used for therapeutic purpose as immunomodulatory agent.





Similar content being viewed by others
References
Sutherland IW (1996) Extracellular polysaccharides. Biotechnol Prod Prim Metab. https://doi.org/10.1002/9783527620999.ch16f
Borchers AT, Krishnamurthy A, Keen CL, Meyers FJ, Gershwin ME (2008) The immunobiology of mushrooms. Exp Biol Med 233:259–276. https://doi.org/10.3181/0708-MR-227
Zhang Q, Li N, Zhou G, Lu X, Xu Z, Li Z (2003) In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacol Res 48:151–155. https://doi.org/10.1016/S1043-6618(03)00103-8
de Jesus Raposo MF, de Morais AMB, de Morais RMSC (2015) Marine polysaccharides from algae with potential biomedical applications. Mar Drugs 13:2967–3028. https://doi.org/10.3390/md13052967
Guzman S, Gato A, Lamela M, Freire-Garabal M, Calleja J (2003) Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res 17:665–670. https://doi.org/10.1002/ptr.1227
Ferreira SS, Passos CP, Madureira P, Vilanova M, Coimbra MA (2015) Structure–function relationships of immunostimulatory polysaccharides: a review. Carbohydr Polym 132:378–396. https://doi.org/10.1016/j.carbpol.2015.05.079
Zhao G, Kan J, Li Z, Chen Z (2005) Characterization and immunostimulatory activity of an (1 → 6)-aD-glucan from the root of Ipomoea batatas. Int Immunopharmacol 5:1436–1445. https://doi.org/10.1016/j.intimp.2005.03.012
Nosáľová G, Prisenžňáková L, Paulovičová E, Capek P, Matulová M, Navarini L, Liverani FS (2011) Antitussive and immunomodulating activities of instant coffee arabinogalactan-protein. Int J Biol Macromol 49:493–497. https://doi.org/10.1016/j.ijbiomac.2011.06.004
Borowitzka MA (1990) The mass culture of Dunaliella salina. In: Regional workshop on the culture and utilization of seaweeds, Cebu City (Philippines), 27–31 Aug 1990
de Morais MG, Vaz BdS, de Morais EG, Costa JAV (2015) Biologically active metabolites synthesized by microalgae. Biomed Res Int. https://doi.org/10.1155/2015/835761
Bobrov Z, Tracton I, Taunton K, Mathews M (2008) Effectiveness of whole dried Dunaliella salina marine microalgae in the chelating and detoxification of toxic minerals and heavy metals. J Altern Complement Med 14:S8–S9
Ben-Amotz A (1980) Glycerol production in the alga Dunaliella. In: Pietro AS (ed) Biochemical and photosynthetic aspects of energy production. Academia Press Inc, New York, pp 191–208
Ben-Amotz A (1995) New mode of Dunaliella biotechnology: two-phase growth for β-carotene production. J Appl Phycol 7:65–68
Mishra A, Jha B (2009) Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Bioresour Technol 100:3382–3386. https://doi.org/10.1016/j.biortech.2009.02.006
Dai J, Wu Y, Chen S-w, Zhu S, Yin H-p, Wang M, Tang J (2010) Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr Polym 82:629–635. https://doi.org/10.1016/j.carbpol.2010.05.029
Singh P, Baranwal M, Reddy SM (2016) Antioxidant and cytotoxic activity of carotenes produced by Dunaliella salina under stress. Pharm Biol 54:2269–2275. https://doi.org/10.3109/13880209.2016.1153660
Starr RC (1987) UTEX-The culture collection of algae at the University of Texas at Austin. J Phycol 23:1–47
Link AJ, LaBaer J (2011) Trichloroacetic acid (TCA) precipitation of proteins. Cold Spring Harbor Protoc 2011:pdb. prot5651
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S-I, Lee YC (2005) Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal Biochem 339:69–72. https://doi.org/10.1016/j.ab.2004.12.001
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Aziz MA (2015) Qualitative phytochemical screening and evaluation of anti-inflammatory, analgesic and antipyretic activities of Microcos paniculata barks and fruits. J Integr Med 13:173–184. https://doi.org/10.1016/S2095-4964(15)60179-0
Andriamanantoanina H, Chambat G, Rinaudo M (2007) Fractionation of extracted Madagascan Gracilaria corticata polysaccharides: structure and properties. Carbohydr Polym 68:77–88. https://doi.org/10.1016/j.carbpol.2006.07.023
Lohia N, Baranwal M (2017) Immune response of highly conserved influenza A virus matrix 1 peptides. Microbiol Immunol 61:225–231. https://doi.org/10.1111/1348-0421.12485
Vasundhara M, Baranwal M, Kumar A (2016) Fusarium tricinctum, an endophytic fungus exhibits cell growth inhibition and antioxidant activity. Indian J Microbiol 56:433–438. https://doi.org/10.1007/s12088-016-0600-x
Pandey R, Maurya R, Singh G, Sathiamoorthy B, Naik S (2005) Immunosuppressive properties of flavonoids isolated from Boerhaavia diffusa Linn. Int Immunopharmacol 5:541–553. https://doi.org/10.1016/j.intimp.2004.11.001
Guo X, Wang X, Liu J (2016) Composition analysis of fractions of extracellular polymeric substances from an activated sludge culture and identification of dominant forces affecting microbial aggregation. Sci Rep 6:28391. https://doi.org/10.1038/srep28391
Ben-Amotz A, Avron M (1989) The biotechnology of mass culturing Dunaliella for products of commercial interest. Algal Cyanobacterial Biotechnol 91–114
Ray B, Lahaye M (1995) Cell-wall polysaccharides from the marine green alga Ulva “rigida”(Ulvales, Chlorophyta). Chemical structure of ulvan. Carbohydr Res 274:313–318. https://doi.org/10.1016/0008-6215(95)00407-6
Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Bailey T, Longvah T (2013) Immunomodulatory activities of polysaccharides isolated from Taxillus chinensis and Uncaria rhyncophylla. Carbohydr Polym 98:1458–1465. https://doi.org/10.1016/j.carbpol.2013.07.060
Xu X, Tong W, Lei Y, Yao S (2007) Separation and purification of extracellular polysaccharides from Dunaliella salina. J Food Sci Biotechnol 26:28–33
Idriss HT, Naismith JH (2000) TNFα and the TNF receptor superfamily: structure-function relationship (s). Microsc Res Tech 50:184–195. https://doi.org/10.1002/1097-0029(20000801)50:3%3c184:AID-JEMT2%3e3.0.CO;2-H
Maspi N, Abdoli A, Ghaffarifar F (2016) Pro-and anti-inflammatory cytokines in cutaneous leishmaniasis: a review. Pathog Glob Health 110:247–260. https://doi.org/10.1080/20477724.2016.1232042
Schoenborn JR, Wilson CB (2007) Regulation of interferon-γ during innate and adaptive immune responses. Adv Immunol 96:41–101. https://doi.org/10.1016/S0065-2776(07)96002-2
Huber S, Schramm C (2006) TGF-beta and CD4+ CD25+ regulatory T cells. Front Biosci 11:1014–1023
Li M, Yan YX, Yu QT, Deng Y, Wu DT, Wang Y, Ge YZ, Li SP, Zhao J (2017) Comparison of immunomodulatory effects of fresh garlic and black garlic polysaccharides on RAW 264.7 Macrophages. J Food Sci 82:765–771. https://doi.org/10.1111/1750-3841.13589
Wu J, Li M, Liu L, An Q, Zhang J, Zhang J, Li M, Duan W, Liu D, Li Z (2013) Nitric oxide and interleukins are involved in cell proliferation of RAW 264. 7 macrophages activated by viili exopolysaccharides. Inflammation 36:954–961. https://doi.org/10.1007/s10753-013-9626-y
Chang C-Y, Tucci M, Baker RC (2000) Lipopolysaccharide-stimulated nitric oxide production and inhibition of cell proliferation is antagonized by ethanol in a clonal macrophage cell line. Alcohol 20:37–43. https://doi.org/10.1016/S0741-8329(99)00054-3
Moore RN, Steeg PS, Männel DN, Mergenhagen SE (1980) Role of lipopolysaccharide in regulating colony-stimulating factor-dependent macrophage proliferation in vitro. Infect Immun 30:797–804
Zhuang JC, Wogan GN (1997) Growth and viability of macrophages continuously stimulated to produce nitric oxide. Proc Natl Acad Sci 94:11875–11880. https://doi.org/10.1073/pnas.94.22.11875
Zhu L, Cao J, Chen G, Xu Y, Lu J, Fang F, Chen K (2016) Anti-tumor and immunomodulatory activities of an exopolysaccharide from Rhizopus nigricans on CT26 tumor-bearing mice. Int Immunopharmacol 36:218–224. https://doi.org/10.1016/j.intimp.2016.04.033
Shin JS, Jung JY, Lee SG, Shin KS, Rhee YK, Lee MK, Hong HD, Lee KT (2016) Exopolysaccharide fraction from Pediococcus pentosaceus KFT 18 induces immunostimulatory activity in macrophages and immunosuppressed mice. J Appl Microbiol 120:1390–1402. https://doi.org/10.1111/jam.13099
Mishra A, Kavita K, Jha B (2011) Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr Polym 83:852–857. https://doi.org/10.1016/j.biortech.2009.02.006
Acknowledgements
The author would like to acknowledge Dr. M. Krishnamohan, Birla Institute of Scientific Research, Jaipur, India for providing the culture of Dunaliella salina and Dr. Akshey Jain from Nitin Nursing Home and Dr. Vandana Goyal, from Rajendra Hospital, Patiala for providing us the blood needed for carrying out the experiments. The authors also would like acknowledge Mr. Anoop Patiyal from SAIF, Punjab University, Chandigarh for mass spectroscopic analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial interests exist.
Informed Consent
The informed consent was given by all the donors for the experiments.
Research Involving Human Participants and/or Animals
The study obtained the approval from the institutional ethical committee (IEC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goyal, M., Baranwal, M., Pandey, S.K. et al. Hetero-Polysaccharides Secreted from Dunaliella salina Exhibit Immunomodulatory Activity Against Peripheral Blood Mononuclear Cells and RAW 264.7 Macrophages. Indian J Microbiol 59, 428–435 (2019). https://doi.org/10.1007/s12088-019-00818-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-019-00818-w