Abstract
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States, with high associated costs. Most of the cost burden results from acute exacerbations of COPD (AE-COPD), events associated with heightened symptoms and mortality. Cellular mechanisms underlying AE-COPD are poorly understood, likely because they arise from dysregulation of complex immune networks across multiple tissue compartments.
Methods
To gain systems-level insight into cellular environments relevant to exacerbation, we applied data-driven modeling approaches to measurements of immune factors (cytokines and flow cytometry) measured previously in two different human tissue environments (sputum and peripheral blood) during the stable and exacerbated state.
Results
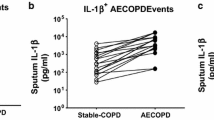
Using partial least squares discriminant analysis, we identified a unique signature of cytokines in serum that differentiated stable and AE-COPD better than individual measurements. Furthermore, we found that models integrating data across tissue compartments (serum and sputum) trended towards being more accurate. The resulting paracrine signature defining AE-COPD events combined elevations of proteins associated with cell adhesion (sVCAM-1, sICAM-1) and increased levels of neutrophils and dendritic cells in blood with elevated chemoattractants (IP-10 and MCP-2) in sputum.
Conclusions
Our results supported a new hypothesis that AE-COPD is driven by immune cell trafficking into the lung, which requires expression of cell adhesion molecules and raised levels of innate immune cells in blood, with parallel upregulated expression of specific chemokines in pulmonary tissue. Overall, this work serves as a proof-of-concept for using data-driven modeling approaches to generate new insights into cellular processes involved in complex pulmonary diseases.





Similar content being viewed by others
References
Aaron, C. P., J. E. Schwartz, S. J. Bielinski, E. A. Hoffman, J. H. M. Austin, E. C. Oelsner, K. M. Donohue, R. Kalhan, C. Berardi, J. D. Kaufman, D. R. Jacobs, R. P. Tracy, and R. G. Barr. Intercellular adhesion molecule 1 and progression of percent emphysema: The MESA Lung Study. Resp. Med. 109:255–264, 2015.
Agouridakis, P., D. Kyriakou, M. G. Alexandrakis, A. Prekates, K. Perisinakis, N. Karkavitsas, and D. Bouros. The predictive role of serum and bronchoalveolar lavage cytokines and adhesion molecules for acute respiratory distress syndrome development and outcome. Resp. Res. 3:25–25, 2002.
Agustí, A., L. D. Edwards, S. I. Rennard, W. MacNee, R. Tal-Singer, B. E. Miller, J. Vestbo, D. A. Lomas, P. M. A. Calverley, E. Wouters, C. Crim, J. C. Yates, E. K. Silverman, H. O. Coxson, P. Bakke, R. J. Mayer, B. Celli, and ECLIPSE Investigators. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS ONE 7:e37483–e37483, 2012.
Albert, R. K., J. Connett, W. C. Bailey, R. Casaburi, J. A. D. Cooper, G. J. Criner, J. L. Curtis, M. T. Dransfield, M. K. Han, S. C. Lazarus, B. Make, N. Marchetti, F. J. Martinez, N. E. Madinger, C. McEvoy, D. E. Niewoehner, J. Porsasz, C. S. Price, J. Reilly, P. D. Scanlon, F. C. Sciurba, S. M. Scharf, G. R. Washko, P. G. Woodruff, N. R. Anthonisen, and C. C. R. Network. Azithromycin for prevention of exacerbations of COPD. New Engl. J. Med. 365:689–698, 2011.
Andelid, K., A. Andersson, S. Yoshihara, C. Âhrén, P. Jirholt, A. Ekberg-Jansson, and A. Lindén. Systemic signs of neutrophil mobilization during clinically stable periods and during exacerbations in smokers with obstructive pulmonary disease. Int. J. Chronic Obstr. 10:1253–1263, 2015.
Arnold, K. B., A. Burgener, K. Birse, L. Romas, L. J. Dunphy, K. Shahabi, M. Abou, G. R. Westmacott, S. McCorrister, J. Kwatampora, B. Nyanga, J. Kimani, L. Masson, L. J. Liebenberg, S. S. Abdool Karim, J.-A. S. Passmore, D. A. Lauffenburger, R. Kaul, and L. R. McKinnon. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. 9:194–205, 2016.
Bafadhel, M., S. McKenna, S. Terry, V. Mistry, C. Reid, P. Haldar, M. McCormick, K. Haldar, T. Kebadze, A. Duvoix, K. Lindblad, H. Patel, P. Rugman, P. Dodson, M. Jenkins, M. Saunders, P. Newbold, R. H. Green, P. Venge, D. A. Lomas, M. R. Barer, S. L. Johnston, I. D. Pavord, and C. E. Brightling. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am. J. Resp. Crit. Care 184:662–671, 2011.
Barnes, P. J. The cytokine network in chronic obstructive pulmonary disease. Am. J. Resp. Cell Mol. 41:631–638, 2009.
Cane, J. L., B. Mallia-Millanes, D. L. Forrester, A. J. Knox, C. E. Bolton, and S. R. Johnson. Matrix metalloproteinases -8 and -9 in the airways, blood and urine during exacerbations of COPD. COPD 13:26–34, 2016.
Chakrabarti, S., and K. D. Patel. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J. Leukocyte Biol. 78:279–288, 2005.
Chang, C., Z. Guo, N. Shen, B. He, W. Yao, H. Zhu, and J. Zhao. Dynamics of inflammation resolution and symptom recovery during AECOPD treatment. Sci. Rep. 4:5516–5516, 2014.
Chen, Y.-W. R., J. M. Leung, and D. D. Sin. A systematic review of diagnostic biomarkers of COPD exacerbation. PLoS ONE 11:e0158843–e0158843, 2016.
Cosio, M. G., M. Saetta, and A. Agustí. Immunologic aspects of chronic obstructive pulmonary disease. New Engl. J. Med. 360:2445–2454, 2009.
Curtis, J. L., C. M. Freeman, and J. C. Hogg. The immunopathogenesis of chronic obstructive pulmonary disease: Insights from recent research. Proc. Am. Thorac. Soc. 4:512–521, 2007.
Dentener, M. A., E. C. Creutzberg, A. M. Schols, A. Mantovani, C. van’t Veer, W. A. Buurman, and E. F. Wouters. Systemic anti-inflammatory mediators in COPD: increase in soluble interleukin 1 receptor II during treatment of exacerbations. Thorax 56:721–726, 2001.
Dinarello, C. A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117:3720–3732, 2011.
Douni, E., and G. Kollias. A critical role of the p75 tumor necrosis factor receptor (p75TNF-R) in organ inflammation independent of TNF, lymphotoxin alpha, or the p55TNF-R. J. Exp. Med. 188:1343–1352, 1998.
El-Deek, S. E., H. A. Makhlouf, T. H. Saleem, M. A. Mandour, and N. A. Mohamed. Surfactant protein D, soluble intercellular adhesion molecule-1 and high-sensitivity C-reactive protein as biomarkers of chronic obstructive pulmonary disease. Med. Prin. Pract. 22:469–474, 2013.
Ford, E. S., L. B. Murphy, O. Khavjou, W. H. Giles, J. B. Holt, and J. B. Croft. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest 147:31–45, 2015.
Freeman, C. M., and J. L. Curtis. Lung dendritic cells: shaping immune responses throughout COPD progression. Am. J. Resp. Cell Mol. 56:152–159, 2017.
Freeman, C. M., F. J. Martinez, M. K. Han, T. M. Ames, S. W. Chensue, J. C. Todt, D. A. Arenberg, C. A. Meldrum, C. Getty, L. McCloskey, and J. L. Curtis. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am. J. Resp. Crit. Care 180:1179–1188, 2009.
Freeman, C. M., C. H. Martinez, J. C. Todt, F. J. Martinez, M. K. Han, D. L. Thompson, L. McCloskey, and J. L. Curtis. Acute exacerbations of chronic obstructive pulmonary disease are associated with decreased CD4+ & CD8+ T cells and increased growth & differentiation factor-15 (GDF-15) in peripheral blood. Resp. Res. 16:94–94, 2015.
Gerritsen, W. B. M., J. Asin, P. Zanen, J. M. M. van den Bosch, and F. J. L. M. Haas. Markers of inflammation and oxidative stress in exacerbated chronic obstructive pulmonary disease patients. Resp. Med. 99:84–90, 2005.
Groenewegen, K. H., M. A. Dentener, and E. F. M. Wouters. Longitudinal follow-up of systemic inflammation after acute exacerbations of COPD. Resp. Med. 101:2409–2415, 2007.
Halpin, D. M. G., M. Miravitlles, N. Metzdorf, and B. Celli. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int. J. Chronic. Obstr. 2891–2908:2017, 2017.
Hollander, C., B. Sitkauskiene, R. Sakalauskas, U. Westin, and S. M. Janciauskiene. Serum and bronchial lavage fluid concentrations of IL-8, SLPI, sCD14 and sICAM-1 in patients with COPD and asthma. Resp. Med. 101:1947–1953, 2007.
Hunter, C. A., and S. A. Jones. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16:448–457, 2015.
Hurst, J. R., G. C. Donaldson, W. R. Perera, T. M. A. Wilkinson, J. A. Bilello, G. W. Hagan, R. S. Vessey, and J. A. Wedzicha. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am. J. Resp. Crit. Care 174:867–874, 2006.
Johannesdottir, S. A., C. F. Christiansen, M. B. Johansen, M. Olsen, X. Xu, J. M. Parker, N. A. Molfino, T. L. Lash, and J. P. Fryzek. Hospitalization with acute exacerbation of chronic obstructive pulmonary disease and associated health resource utilization: a population-based Danish cohort study. J. Med. Econ. 16:897–906, 2013.
Karadag, F., A. B. Karul, O. Cildag, M. Yilmaz, and H. Ozcan. Biomarkers of systemic inflammation in stable and exacerbation phases of COPD. Lung 186:403–409, 2008.
Kochanek, K. D., S. Murphy, J. Xu, and E. Arias. Mortality in the United States, 2016. NCHS Data Brief 1–8, 2017.
Krommidas, G., K. Kostikas, G. Papatheodorou, A. Koutsokera, K. I. Gourgoulianis, C. Roussos, N. G. Koulouris, and S. Loukides. Plasma leptin and adiponectin in COPD exacerbations: associations with inflammatory biomarkers. Resp. Med. 104:40–46, 2010.
Kwiatkowska, S., K. Noweta, M. Zieba, D. Nowak, and P. Bialasiewicz. Enhanced exhalation of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in patients with COPD exacerbation: a prospective study. Respiration 84:231–241, 2012.
Lau, K. S., A. M. Juchheim, K. R. Cavaliere, S. R. Philips, D. A. Lauffenburger, and K. M. Haigis. In vivo systems analysis identifies spatial and temporal aspects of the modulation of TNF-α-induced apoptosis and proliferation by MAPKs. Sci. Signal. 4:ra16, 2011.
Leeuwenberg, J. F., E. F. Smeets, J. J. Neefjes, M. A. Shaffer, T. Cinek, T. M. Jeunhomme, T. J. Ahern, and W. A. Buurman. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology 77:543–549, 1992.
Liu, M., S. Guo, J. M. Hibbert, V. Jain, N. Singh, N. O. Wilson, and J. K. Stiles. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 22:121–130, 2011.
Martinez, F. J., P. M. A. Calverley, U.-M. Goehring, M. Brose, L. M. Fabbri, and K. F. Rabe. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 385:857–866, 2015.
Masciantonio, M. G., C. K. S. Lee, V. Arpino, S. Mehta, and S. E. Gill. The balance between metalloproteinases and TIMPs. Prog. Mol. Biol. Transl. Sci. 147:101–131, 2017.
McCubbrey, A. L., J. Sonstein, T. M. Ames, C. M. Freeman, and J. L. Curtis. Glucocorticoids relieve collectin-driven suppression of apoptotic cell uptake in murine alveolar macrophages through downregulation of SIRPα. J. Immunol. 189:112–119, 2012.
Miller, B. E., R. Tal-Singer, S. I. Rennard, A. Furtwaengler, N. Leidy, M. Lowings, U. J. Martin, T. R. Martin, D. D. Merrill, J. Snyder, J. Walsh, and D. M. Mannino. Plasma fibrinogen qualification as a drug development tool in chronic obstructive pulmonary disease. Perspective of the chronic obstructive pulmonary disease biomarker qualification consortium. Am. J. Resp. Crit. Care 193:607–613, 2016.
Mishra, A., Y. Guo, L. Zhang, S. More, T. Weng, N. R. Chintagari, C. Huang, Y. Liang, S. Pushparaj, D. Gou, M. Breshears, and L. Liu. A critical role for P2X7 receptor-induced VCAM-1 shedding and neutrophil infiltration during acute lung injury. J. Immunol. 197:2828–2837, 2016.
Müller, B., and M. Tamm. Biomarkers in acute exacerbation of chronic obstructive pulmonary disease: among the blind, the one-eyed is king. Am. J. Resp. Crit. Care 174:848–849, 2006.
Navratilova, Z., V. Kolek, and M. Petrek. Matrix metalloproteinases and their inhibitors in chronic obstructive pulmonary disease. Arch. Immunol. Ther. Exerc. 64:177–193, 2016.
O’Dwyer, D. N., K. C. Norman, M. Xia, Y. Huang, S. J. Gurczynski, S. L. Ashley, E. S. White, K. R. Flaherty, F. J. Martinez, S. Murray, I. Noth, K. B. Arnold, and B. B. Moore. The peripheral blood proteome signature of idiopathic pulmonary fibrosis is distinct from normal and is associated with novel immunological processes. Sci. Rep. 7:46560–46560, 2017.
Oba, Y., and N. A. Lone. Efficacy and safety of roflumilast in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ther. Adv. Respir. Dis. 7:13–24, 2013.
Papi, A., C. M. Bellettato, F. Braccioni, M. Romagnoli, P. Casolari, G. Caramori, L. M. Fabbri, and S. L. Johnston. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Resp. Crit. Care 173:1114–1121, 2006.
Peters, V. A., J. J. Joesting, and G. G. Freund. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 32:1–8, 2013.
Roche, N., M. Zureik, D. Soussan, F. Neukirch, D. Perrotin, and B. S. C. Urgence and Investigators. Predictors of outcomes in COPD exacerbation cases presenting to the emergency department. Eur. Respir. J. 32:953–961, 2008.
Rodriguez-Roisin, R. Toward a consensus definition for COPD exacerbations. Chest 117:398S–401S, 2000.
Röpcke, S., O. Holz, G. Lauer, M. Müller, S. Rittinghausen, P. Ernst, G. Lahu, M. Elmlinger, N. Krug, and J. M. Hohlfeld. Repeatability of and relationship between potential COPD biomarkers in bronchoalveolar lavage, bronchial biopsies, serum, and induced sputum. PLoS ONE 7:e46207–e46207, 2012.
Santos, S., A. Marín, J. Serra-Batlles, D. de la Rosa, I. Solanes, X. Pomares, M. López-Sánchez, M. Muñoz-Esquerre, and M. Miravitlles. Treatment of patients with COPD and recurrent exacerbations: the role of infection and inflammation. Int. J. Chronic Obstr. 11:515–525, 2016.
Thomsen, M., T. S. Ingebrigtsen, J. L. Marott, M. Dahl, P. Lange, J. Vestbo, and B. G. Nordestgaard. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 309:2353–2361, 2013.
Toy, E. L., K. F. Gallagher, E. L. Stanley, A. R. Swensen, and M. S. Duh. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD 7:214–228, 2010.
Vaitkus, M., S. Lavinskiene, D. Barkauskiene, K. Bieksiene, J. Jeroch, and R. Sakalauskas. Reactive oxygen species in peripheral blood and sputum neutrophils during bacterial and nonbacterial acute exacerbation of chronic obstructive pulmonary disease. Inflammation 36:1485–1493, 2013.
Wedzicha, J. A., D. Banerji, K. R. Chapman, J. Vestbo, N. Roche, R. T. Ayers, C. Thach, R. Fogel, F. Patalano, C. F. Vogelmeier, and F. Investigators. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. New Engl. J. Med. 374:2222–2234, 2016.
Witkowska, A. M., and M. H. Borawska. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur. Cytokine Netw. 15:91–98, 2004.
Wold, S., E. Johansson, and M. Cocchi. PLS-partial least squares projections to latent structures. In: 3D QSAR in Drug Design: Theory Methods and Applications, edited by H. Kubinyi. Dordrecht: Escom, 1993, pp. 523–550.
Xu, X., P. L. Jackson, S. Tanner, M. T. Hardison, M. A. Roda, J. E. Blalock, and A. Gaggar. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS ONE 6:e15781, 2011.
Yung S. C. and J. M. Farber. Chemokines. edited by A. J. KastinElsevier, pp. 656–663, 2013.
Acknowledgments
The authors would like to thank Lisa McCloskey, RRT, Christi Getty, RRT, and Candace Flaherty, RRT for interactions with subjects in the original study.
Funding
This work was supported by NIH R01 HL144849-01 (to K.B.A.). K.C.N. was supported by a Department of Education Graduate Assistance in Areas of National Need (GAANN) Fellowship awarded to the biomedical engineering department at the University of Michigan (PR Award Number: P200A150170). C.M.F. was supported by Merit Review Awards I01 CX001553 from the Department of Veterans Affairs and by MedImmune, Ltd. M.K.H. reports a grant from the National Heart, Lung and Blood Institute. F.J.M. has received grants from the National Institute of Health. J.L.C. was supported by Merit Review Awards I01 CX000911 from the Department of Veterans Affairs and by MedImmune, Ltd.
Conflict of interest
K.C.N., C.M.F., N.S.B, J.L.C. and K.B.A. reported no conflicts of interest. M.K.H. reports consultant arrangements with GlaxoSmithKline, Boehringer Ingelheim, Novartis, Sunovion, and AstraZeneca. F.J.M. has received personal fees from Forest, Janssen, GlaxoSmithKline, Nycomed/Takeda, Amgen, AstraZeneca, Boehringer Ingelheim, Ikaria/Bellerophon, Genentech, Novartis, Pearl, Pfizer, Roche, Sunovion, Theravance, Axon, CME Incite, California Society for Allergy and Immunology, Annenberg, Integritas, InThough, Miller Medical, National Association for Continuing Education, Paradigm, Peer Voice, UpToDate, Haymarket Communications, Western Society of Allergy and Immunology, Informa, Bioscale, Unity Biotechnology, ConCert, Lucid, Methodist Hospital, Prime, WebMD, Bayer, Ikaria, Kadmon, Vercyte, American Thoracic Society, Academic CME, Falco, Axon Communication, Johnson & Johnson, Clarion, Continuing Education, Potomac, Afferent, and Adept; and has collected nonfinancial support from Boehringer Ingelheim, Centocor, Gilead, and Biogen/Stromedix; and declares other interests with Mereo, Boehringering Ingelheim, and Centocor.
Ethical Standards
All human subjects research was carried out in accordance with the Declaration of Helsinki and were approved by the Institutional Review Boards of the Veterans’ Affairs Ann Arbor Healthcare System (VAAHS) and of the University of Michigan Health System (UMHS).
Research Involved in Human or animal rights
No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor William H. Guilford oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Norman, K.C., Freeman, C.M., Bidthanapally, N.S. et al. Inference of Cellular Immune Environments in Sputum and Peripheral Blood Associated with Acute Exacerbations of COPD. Cel. Mol. Bioeng. 12, 165–177 (2019). https://doi.org/10.1007/s12195-019-00567-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-019-00567-2