Abstract
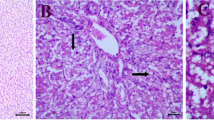
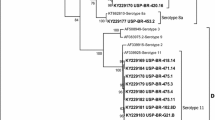
Inclusion body hepatitis (IBH), hydropericardium syndrome (HS), and gizzard erosion (GE) are all economically important diseases in the poultry industry worldwide and are all caused by fowl aviadenovirus (FAdV). It is important to identify the serotype of the virus to differentiate these diseases. In the present study, a total of six recent FAdV serotypes were isolated and identified in broiler and broiler-breeder flocks in Izmir, Manisa, and Aydın provinces of the Aegean region of Turkey between January and March 2019. The viruses were isolated from livers and pooled organs of chickens using primary chicken embryo kidney cell cultures (CEKC). Virus isolates were identified by PCR amplification of the loop 1 (L1) variable region of the hexon gene followed by Sanger sequencing. Sequence analysis revealed the presence of both FAdV-D (serotype 11) and FAdV-E (serotype 8b). The viruses that were isolated were associated with IBH, which is typically characterized by gross lesions such as enlarged and pale yellow liver with multiple petechial hemorrhages. Histopathological examination also showed necrotizing hepatitis with intranuclear inclusion bodies in hepatocytes. This study is the first report of the isolation and identification of FAdV serotypes associated with IBH in commercial broilers and broiler-breeder flocks in Turkey. The results of sequence analysis showed that FAdV-8b and FAdV-11 were the circulating serotypes that caused recent field outbreaks of IBH in the Aegean region between January and March, 2019.





Similar content being viewed by others
Change history
27 November 2019
The co-author names were found missed in the original publication of this article and the complete lists of authors were updated here. The original article has been corrected.
References
Adair BM, McFerran JB (2008) Adenoviruses in a laboratory manual for the isolation, identification and characterization of avian pathogens, 5th edn. In: Zavala LD, Swayne DE, Glisson JR, Pearson JE, Reed WM, Jackwood MW, Woolcock PR (eds) A laboratory manual for the isolation, identification, and characterization of avian pathogens. The American Association of Avian Pathologists, Kennett Square, pp 84–89
Adair BM, Smyth JA (2008) Group I Adenovirus infections. In: Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE (eds) Diseases of poultry, 12th edn. Iowa State University Press, Iowa, pp 252–266
Ahmad K, Ahmad I, Muneer MA, Ajmal M (1992) Experimental transmission of Angara disease in broiler fowls. Stud Res Vet Med 1:53–55
Akhtar S, Zahid S, Khan MI (1992) Risk factors associated with hydropericardium syndrome in broiler flocks. Vet Rec 131:481–484
Akhtar S (1995) Lateral spread of the aetiologic agent(s) of hydropericardium syndrome in broiler chickens. Vet Rec 136:118–120
Choi K, Kye SJ, Kim JY, Jeon WJ, Lee EK, Park KY, Sung HW (2012) Epidemiological investigation of outbreaks of fowl adenovirus infection in commercial chickens in Korea. Poult Sci 91:2502–2506
Cook JKA (1974) Spread of an avian adenovirus (CELOvirus) to uninoculated fowls. Res Vet Sci 16:156–161
Dar A, Gomis S, Shirley I, Mutwiri G, Brownlie R, Potter A, Gerdts V, Tikoo SK (2012) Pathotypic and molecular characterization of a fowl adenovirus associated with inclusion body hepatitis in Saskatchewan chickens. Avian Dis 56:73–81
Helmboldt CF, Frazier MN (1963) Avian hepatic inclusion bodies of unknown significance. Avian Dis 7(4):446–450
Hess M, Raue R, Prusas C (1999) Epidemiological studies on fowl adenoviruses isolated from cases of infectious hydropericardium. Avian Pathol 28:433–439
Hess M (2000) Detection and differentiation of avian adenoviruses: a review. Avian Pathol 29:195–206
Hess M, Prusas C, Bergmann V, Mazaheri A, Raue R (2000) Epizootiology of fowls adenoviruses Berl. Munch Tierarztl Wochenschr 113:202–208
Hess M (2013) Diseases of poultry. Aviadenovirus infections, 13th edn. Wiley, Hoboken, pp 290–300
Howell J, MacDonald DW, Christian RG (1970) Inclusion body hepatitis in chickens. Can Vet J 11(5):99–101
Kataria JM, Dhama K, Nagarajan S, Chakraborty S, Kaushal A, Deb R (2013) Fowl adenoviruses causing hydropericardium syndrome in poultry. Adv Anim Vet Sci 1(4):5–13
Lim TH, Lee HJ, Lee DH, Lee YN, Park JK, Youn HN, Kim MS, Youn HS, Lee JB, Park SY, Choi IS, Song CS (2011) Identification and virulence characterization of fowl adenoviruses in Korea. Avian Dis 55:554–560
Macpherson I, McDougall JS, Laursen-Jones AP (1974) Inclusion body hepatitis in a broiler integration. Vet Rec 95(13):286–289
Mase M, Nakamura K, Minami F (2012) Fowl adenoviruses isolated from chickens with inclusion body hepatitis in Japan, 2009–2010. J Vet Med Sci 74:1087–1089
Matos M, Grafl B, Liebhart D, Hess M (2016) The outcome of experimentally induced inclusion body hepatitis (IBH) by fowl aviadenoviruses (FAdVs) is crucially influenced by the genetic background of the host. Vet Res 47:69
Mazaheri A, Prusas C, Vossand M, Hess M (2003) Vertical transmission of fowl Adenovirus serotype 4 investigated in specified pathogen-free birds after experimental infection. Archiv für Geflügelkunde 67:6–10
McFerran JB, McCracken RM, Connor TJ, Evans RT (1976) Isolation of viruses from clinical outbreaks of inclusion body hepatitis. Avian Pathol 5(4):315–324
Meulemans G, Boschmans M, Van den Berg TP, Decaesstecker M (2001) Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathol 30(6):655–660. https://doi.org/10.1080/03079450120092143
Morshed R, Hosseini H, Langeroudi AG, Fard MHB, Charkhkar S (2017) Fowl adenoviruses D and E cause inclusion body hepatitis outbreaks in broiler and broiler-breeder pullet flocks. Avian Dis 61:205–210
Nakamura K, Mase M, Yamamoto Y, Takizawa K, Kabeya M, Wakuda T, Matsuda M, Chikuba T, Yamamoto Y, Ohyama T, Takahashi K, Sato N, Akiyama N, Honma H, Imai K (2011) Inclusion body hepatitis caused by fowl adenovirus in broiler chickens in Japan, 2009–2010. Avian Dis 55:719–723
Ojkic D, Martin E, Swinton J, Vaillancourt JP, Boulianne M, Gomis S (2008) Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathol 37:95–100
Raue R, Hess M (1998) Hexon based PCRs combined with restriction enzyme analysis for rapid detection and differentiation of fowl adenoviruses and egg drop syndrome virus. J Virol Methods 73:211–217
Sanei B (2009) IBH in ontario broilers. Canadian poultry magazine. Canadianpoultrymag.com, Ontario, pp 22–28
Senties-Cue CG et al (2010) Epidemiology and effect on production parameters of an outbreak of inclusion body hepatitis in broilers. Avian Dis 54(1):74–78
Steer PA, O’Rourke D, Ghorashi SA, Noormohammadi AH (2011) Application of high-resolution melting curve analysis for typing of fowl adenoviruses in field cases of inclusion body hepatitis. Aust Vet J 89:184–192
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Toro H, González O, Escobar C, Cerda L, Morales MA, González C (2001) Vertical induction of the inclusion body hepatitis/hydropericardium syndrome with fowl adenovirus and chicken anemia virus. Avian Dis 45:215–222
Toro H, Gonzalez C, Cerda L, Morales MA, Dooner P, Salamero M (2002) Prevention of inclusion body hepatitis/hydropericardium syndrome in progeny chickens by vaccination of breeders with fowl adenovirus and chicken anemia virus. Avian Dis 46:547–554
Villegas PC (2006) Laboratory manual avian virus diseases. College of Veterinary Medicine, Athens, pp 5–6
Wilson FD, Wills RW, Senties-cue CG, Maslin WR, Stayer PA, Magee DL (2010) High incidence of glomerulonephritis associated with inclusion body hepatitis in broiler chickens: routine histopathology and histomorphometric studies. Avian Dis 54:975–980
Zadravec M, Slavec B, Krapez U, Kajan GL, Racnik J, Junts P, Cizerl RJ, Benko M, Rojs OZ (2013) Inclusion body hepatitis (IBH) outbreak associated with fowl adenovirus type 8b in broilers. Acta Vet 63:101–110
Zhao J, Zhong Q, Zhao Y, Hu YX, Zhang GZ (2015) Correction: Pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PLoS One 11:e0161744
Acknowledgements
The authors thank Dr. Ozhan Turkyilmaz, Dr. Gulnur Kalayci, Mr. Hasan Huseyin Pala and Mr. Bahtiyar Yılmaz for their contribution and help. The authors also especially thank Dr. Gerald Barry (University College of Dublin, Ireland) for English language editing and scientific advice. This study was supported by the Izmir/Bornova Veterinary Control Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
In this study, the samples were collected after the death of chickens because of virus infections in broiler and broiler-breeder flocks. No chickens were killed for the purpose of the sampling in the present study.
Additional information
Handling Editor: Graciela Andrei.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The co-author names were found missing in the original article and the author names were updated here.
Rights and permissions
About this article
Cite this article
Şahindokuyucu, İ., Çöven, F., Kılıç, H. et al. First report of fowl aviadenovirus serotypes FAdV-8b and FAdV-11 associated with inclusion body hepatitis in commercial broiler and broiler-breeder flocks in Turkey. Arch Virol 165, 43–51 (2020). https://doi.org/10.1007/s00705-019-04449-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-019-04449-w