Abstract
Background
Nivolumab has demonstrated antitumor activity and manageable safety in the single-arm, phase II CheckMate 275 study in patients with unresectable locally advanced or metastatic platinum-resistant urothelial carcinoma. We report updated results of the global population and a subanalysis of Japanese patients from this study.
Methods
Patients received nivolumab 3 mg/kg intravenously every 2 weeks until progression or unacceptable toxicity. The primary endpoint was objective response rate (ORR) confirmed by blinded independent review committee (BIRC) per Response Evaluation Criteria in Solid Tumors v1.1. Secondary endpoints included progression-free survival (PFS) by BIRC and overall survival (OS). Safety was also reported. The minimum follow-up was 21 months.
Results
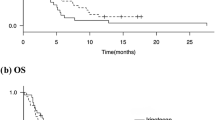
Overall, 270 patients were treated with nivolumab globally; 23 patients were Japanese. In the global and Japanese populations, respectively, ORR per BIRC was 20.4% and 21.7%; median PFS was 1.9 (95% confidence interval [CI] 1.9–2.3) and 3.8 months (95% CI 1.9–7.2); and median OS was 8.6 (95% CI 6.1–11.3) and 21.0 months (95% CI 7.2–not reached). The most common any grade treatment-related adverse events were fatigue (18.1%) and diarrhea (12.2%) in the global population; the most common in the Japanese population were diarrhea (26.1%) and pyrexia (13.0%). Grade 3 or 4 treatment-related adverse events occurred in 61 (22.6%) and seven (30.4%) of the global and Japanese patients, respectively.
Conclusions
Nivolumab continues to show antitumor activity and survival in the global population of CheckMate 275. Meaningful clinical benefit was also observed in Japanese patients. No new safety signals were identified.





Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Ervikm M et al (2013) GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase. http://globocan.iarc.fr/. Accessed 10 May 2018
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Wong MCS, Fung FDH, Leung C et al (2018) The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep 8(1):1129
Kakehi Y, Hirao Y, Kim WJ et al (2010) Bladder cancer working group report. Jpn J Clin Oncol 40(Suppl 1):i57–i64
Klaile Y, Schlack K, Boegemann M et al (2016) Variant histology in bladder cancer: how it should change the management in non-muscle invasive and muscle invasive disease? Transl Androl Urol 5(5):692–701
Ravi K, Kumar T, Bakshi H et al (2013) Non urothelial bladder cancers: a case series. Indian J Surg Oncol 4(1):2–8
Bellmunt J, Orsola A, Leow JJ et al (2014) Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii40–iii48
Kubota Y, Nakaigawa N, Committee for Establishment of the Clinical Practice Guideline for the Management of Bladder Cancer and the Japanese Urological Association (2016) Essential content of evidence-based clinical practice guidelines for bladder cancer: the Japanese Urological Association 2015 update. Int J Urol 23(8):640–645
National Comprehensive Cancer Network (2019) Bladder cancer (version 1.2019). https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf. Accessed 29 Jan 2019
European Medicines Agency; Committee for Medicinal Products for Human Use (CHMP) (2017) Summary of opinion (post authorisation), Opdivo; EMA/CHMP/251204/2017. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/003985/WC500226181.pdf. Accessed 14 June 2018
European Medicines Agency; Committee for Medicinal Products for Human Use (CHMP) (2017) Summary of opinion (post authorisation), Keytruda; EMA/CHMP/428392/2017. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/003820/WC500231699.pdf. Accessed 14 June 2018
Opdivo (nivolumab) injection (2018) [prescribing information]. Bristol-Myers Squibb, Princeton, NJ
Yuasa T, Urakami S, Yonese J (2018) Recent advances in medical therapy for metastatic urothelial cancer. Int J Clin Oncol 23(4):599–607
Sharma P, Retz M, Siefker-Radtke A et al (2017) Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 18(3):312–322
Nozawa M, Nonomura N, Ueda T et al (2013) Adverse event profile and dose modification of everolimus for advanced renal cell carcinoma in real-world Japanese clinical practice. Jpn J Clin Oncol 43(11):1132–1138
Sekine I, Yamamoto N, Nishio K et al (2008) Emerging ethnic differences in lung cancer therapy. Br J Cancer 99(11):1757–1762
Tomita Y, Fukasawa S, Shinohara N et al (2017) Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup analysis from the CheckMate 025 study. Jpn J Clin Oncol 47(7):639–646
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Osoba D, Rodrigues G, Myles J et al (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16(1):139–144
Pickard AS, Neary MP, Cella D (2007) Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 5:70
Bedke J, Sharma P, Retz M et al (2017) CheckMate 275—Phase II studie: update zur wirksamkeit einer nivolumab-monotherapie bei patienten mit einem metastasierten oder inoperablen urothelkarzinom nach platinhaltiger vortherapie. Oncol Res Treat 40(Suppl 3):1–308
Kumar V, Chaudhary N, Garg M et al (2017) Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 8:49
Hida T, Kaji R, Satouchi M et al (2018) Atezolizumab in Japanese patients with previously treated advanced non-small-cell lung cancer: a subgroup analysis of the phase 3 OAK study. Clin Lung Cancer 19(4):e405–e415
Sonpavde G, Pond GR, Choueiri TK et al (2016) Single-agent taxane versus taxane-containing combination chemotherapy as salvage therapy for advanced urothelial carcinoma. Eur Urol 69(4):634–641
Sharma P, Siefker-Radtke A, de Braud F et al (2019) Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J Clin Oncol. https://doi.org/10.1200/JCO.19.00538
Acknowledgements
The authors thank the patients and their families, the investigators and participating study teams, and the Faculty of Medicine, University of Tokyo. Professional medical writing and editorial assistance were provided by Nicolette Belletier, Ph.D, and Lawrence Hargett of PAREXEL, funded by Bristol-Myers Squibb KK and ONO Pharmaceutical Co., Ltd.
Funding
This study was funded by Bristol-Myers Squibb and ONO Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Chikara Ohyama, Takahiro Kojima, Yoshio Naya, Takamitsu Inoue, and Shinichi Hisasue declare having no conflict of interest. Tsunenori Kondo has received honoraria from ONO Pharmaceutical and Pfizer. Yoshihiko Tomita declares an advisory role for Novartis, Taiho Pharmaceutical, and ONO Pharmaceutical, received honoraria from Astellas, ONO Pharmaceutical, Pfizer, Sonafi Aventis, and Bristol-Myers Squibb, and received research funding from Astellas, AstraZeneca, Pfizer, and ONO Pharmaceutical. Masatoshi Eto received honoraria from ONO Pharmaceutical and Bristol-Myers Squibb, and research funding from ONO Pharmaceutical. Hirotsugu Uemura received honoraria from Bayer, AstraZeneca, Takeda, Astellas, Sanofi, Janssen, MSD, Bristol-Myers Squibb, and Pfizer, and received research funding from AstraZeneca, Janssen, Takeda, Astellas, Sanofi, Taiho, ONO Pharmaceutical, and Pfizer. Wataru Obara received honoraria from Bristol-Myers Squibb and research funding from Bristol-Myers Squibb. Eiji Kikuchi received honoraria from ONO Pharmaceutical, Kissei Pharmaceutical, ASKA Pharmaceutical, Nippon Shinyaku Taiho, Takeda, MSD, Pfizer, AstraZeneca, Astellas, and Chugai, and received research funding from MSD, Nippon Kayaku, Astellas, ONO Pharmaceutical, Chugai, Takeda, Taiho, and AstraZeneca. Padmanee Sharma owns stock in Jounce, Neon, Constellation, Oncolytics, BioAtla, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, ImaginAb, and Hummingbird, reports an advisory role in Constellation, Jounce, Neon, BioAtla, Pieris, Oncolytics, Merck, Forty-Seven, Polaris, Apricity, Marker Therapeutics, Codiak, ImaginAb, Hummingbird, and owns a patent licensed to Jounce. Matthew D. Galsky received research funding from Bristol-Myers Squibb, Genentech, AstraZeneca, and Merck. Arlene Siefker-Radtke reports an advisory role for Nektar Therapeutics, Janssen Biotech, Bavarian Nordic, Merck Sharp and Dohme, and Seattle Genetics, and research funding from Janssen, Takeda, Nektar Therapeutics, and Bioclin. Gary Grossfeld reports employment at Bristol-Myers Squibb and owns stock in Bristol-Myers Squibb. Sandra Collette reports employment at Bristol-Myers Squibb. Kyna Gooden reports employment at Bristol-Myers Squibb and owns stock in Bristol-Myers Squibb. Go Kimura received honoraria from ONO Pharmaceutical, Bristol-Myers Squibb, Novartis, Bayer, and Chugai.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ohyama, C., Kojima, T., Kondo, T. et al. Nivolumab in patients with unresectable locally advanced or metastatic urothelial carcinoma: CheckMate 275 2-year global and Japanese patient population analyses. Int J Clin Oncol 24, 1089–1098 (2019). https://doi.org/10.1007/s10147-019-01450-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01450-w