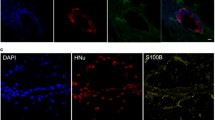
Abstract
Spinal cord injury (SCI) is a common pathological condition that leads to permanent or temporal loss of motor and autonomic functions. Kainic acid (KA), an agonist of kainate receptors, a type of ionotropic glutamate receptor, is widely used to induce experimental neurodegeneration models of CNS. Mesenchymal Stem Cells (MSC) therapy applied at the injured nervous tissue have emerged as a promising therapeutic treatment. Here we used a validated SCI experimental model in which an intraparenchymal injection of KA into the C5 segment of rat spinal cord induced an excitotoxic lesion. Three days later, experimental animals were treated with an intracerebroventricular injection of human umbilical cord (hUC) MSC whereas control group only received saline solution. Sensory and motor skills as well as neuronal and glial reaction of both groups were recorded. Differences in motor behavior, neuronal counting and glial responses were observed between hUC-MSC-treated and untreated rats. According to the obtained results, we suggest that hUC-MSC therapy delivered into the fourth ventricle using the intracerebroventricular via can exert a neuroprotective or neurorestorative effect on KA-injected animals.





Similar content being viewed by others
References
Nicola, F., Marques, M. R., Odorcyk, F., Petenuzzo, L., Aristimunha, D., Vizuete, A., Sanches, E. F., Pereira, D. P., Maurmann, N., Gonçalves, C. A., Pranke, P., & Netto, C. A. (2019). Stem cells from human exfoliated deciduous teeth modulate early astrocyte response after spinal cord contusion. Molecular Neurobiology, 56(1), 748–760. https://doi.org/10.1007/s12035-018-1127-4.
Nejati-Koshki, K., Mortazavi, Y., Pilehvar-Soltanahmadi, Y., Sheoran, S., & Zarghami, N. (2017). An update on application of nanotechnology and stem cells in spinal cord injury regeneration. Biomedicine Pharmacotherapy, 90, 85–92. https://doi.org/10.1016/j.biopha.2017.03.035.
Papa, S., Vismara, I., Mariani, A., Barilani, M., Rimondo, S., De Paola, M., Panini, N., Erba, E., Mauri, E., Rossi, F., Forloni, G., Lazzari, L., & Veglianese, P. (2018). Mesenchymal stem cells encapsulated into biomimetic hydrogel scaffold gradually release CCL2 chemokine in situ preserving cytoarchitecture and promoting functional recovery in spinal cord injury. Journal of Controlled Release, 278, 49–56. https://doi.org/10.1016/j.jconrel.2018.03.034.
Chen, S., Yi, M., Zhou, G., Pu, Y., Hu, Y., Han, M., & Jin, H. (2019). Abdominal aortic transplantation of bone marrow mesenchymal stem cells regulates the expression of ciliary neurotrophic factor and inflammatory cytokines in a rat model of spinal cord ischemia-reperfusion injury. Medical Science Monitor, 25, 1960–1969. https://doi.org/10.12659/MSM.912697.
Reigada, D., Navarro-Ruiz, R. M., Caballero-López, M. J., Del Águila, Á., Muñoz-Galdeano, T., Maza, R. M., & Nieto-Díaz, M. (2017). Diadenosine tetraphosphate (Ap4A) inhibits ATP-induced excitotoxicity: A neuroprotective strategy for traumatic spinal cord injury treatment. Purinergic Signalling, 13, 75–87. https://doi.org/10.1007/s11302-016-9541-4.
Zheng, H., Zhu, W., Zhao, H., Wang, X., Wang, W., & Li, Z. (2010). Kainic acid-activated microglia mediate increased excitability of rat hippocampal neurons in vitro and in vivo: Crucial role of interleukin-1beta. Neuroimmunomodulation, 17, 31–38. https://doi.org/10.1159/000243083.
Kuzhandaivel, A., Nistri, A., & Mladinic, M. (2010). Kainate-mediated excitotoxicity induces neuronal death in the rat spinal cord in vitro via a PARP-1 dependent cell death pathway (Parthanatos). Cellular and Molecular Neurobiology, 30, 1001–1012. https://doi.org/10.1007/s10571-010-9531-y.
Mazzone, L., Margaryan, G., Kuzhandaivel, A., Nasrabady, S. E., Mladinic, M., & Nistri, A. (2010). Kainate-induced delayed onset of excitotoxicity with functional loss unrelated to the extent of neuronal damage in the in vitro spinal cord. Neuroscience, 168, 451–462. https://doi.org/10.1016/j.neuroscience.2010.03.055.
Mitra, N. K., Goh, T. E. W., Krishnan, T. B., Nadarajah, V. D., Vasavaraj, A. K., & Soga, T. (2013). Effect of intra-cisternal application of kainic acid on the spinal cord and locomotor activity in rats. International Journal of Clinical and Experimental Pathology, 6, 1505–1515.
Kjell, J., & Olson, L. (2016). Rat models of spinal cord injury: From pathology to potential therapies. Disease Model Mechanism, 9, 1125–1137. https://doi.org/10.1242/dmm.025833.
Nishida, F., Zanuzzi, C. N., Martínez, A., Barbeito, C. G., & Portiansky, E. L. (2015). Functional and histopathological changes induced by intraparenchymal injection of kainic acid in the rat cervical spinal cord. Neurotoxicology, 49, 68–78. https://doi.org/10.1016/j.neuro.2015.05.006.
Nishida, F., Sisti, M. S., Zanuzzi, C. N., Barbeito, C. G., & Portiansky, E. L. (2017). Neurons of the rat cervical spinal cord express vimentin and neurofilament after intraparenchymal injection of kainic acid. Neuroscience Letters, 64, 103–110. https://doi.org/10.1016/j.neulet.2017.02.029.
Zanuzzi, C. N., Nishida, F., Sisti, S., Barbeito, C. G., & Portiansky, E. L. (2019). Reactivity of microglia and astrocytes after an excitotoxic injury induced by kainic acid in the rat spinal cord. Tissue and Cell, 56, 31–40. https://doi.org/10.1016/j.tice.2018.11.007.
Forostyak, S., Jendelova, P., & Sykova, E. (2013). The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie, 95, 2257–2270. https://doi.org/10.1016/j.biochi.2013.08.004.
Dasari, V. R., Veeravalli, K. K., & Dinh, D. H. (2014). Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World Journal Stem Cells, 6(2), 120–133. https://doi.org/10.4252/wjsc.v6.i2.120.
Vismara, I., Papa, S., Rossi, F., Forloni, G., & Veglianese, P. (2017). Current options for cell therapy in spinal cord injury. Trends in Molecular Medicine, 23, 831–849. https://doi.org/10.1016/j.molmed.2017.07.005.
Cofano, F., Boido, M., Monticelli, M., Zenga, F., Ducati, A., Vercelli, A., & Garbossa, D. (2019). Mesenchymal stem cells for spinal cord injury: Current options, limitations, and future of cell therapy. International Journal Molecular Science, 20(11), pii: E2698. https://doi.org/10.3390/ijms20112698.
Teng, Y. D. (2019). Functional multipotency of stem cells: Biological traits gleaned from neural progeny studies. Seminars in Cell and Developmental Biology, S1084-9521(18), 30059–30054. https://doi.org/10.1016/j.semcdb.2019.02.002.
Boillée, S., Yamanaka, K., Lobsiger, C. S., Copeland, N. G., Jenkins, N. A., Kassiotis, G., Kollias, G., & Cleveland, D. W. (2006). Onset and progression in inherited ALS determined by motor neurons and microglia. Science, 312, 1389–1392. https://doi.org/10.1126/science.1123511.
Lee, H. J., Lee, J. K., Lee, H., Carter, J. E., Chang, J. W., Oh, W., Yang, Y. S., Suh, J. G., Lee, B. H., Jin, H. K., & Bae, J. S. (2012). Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiology of Aging, 33, 588–602. https://doi.org/10.1016/j.neurobiolaging.2010.03.024.
Song, C. G., Zhang, Y. Z., Wu, H. N., Cao, X. L., Guo, C. J., Li, Y. Q., Zheng, M. H., & Han, H. (2018). Stem cells: A promising candidate to treat neurological disorders. Neural Regeneration Research, 13, 1294–1304. https://doi.org/10.4103/1673-5374.235085.
Zappa Villar, M. F., Lehmann, M., Garcia, M. G., Mazzolini, G., Gustavo, R., Morel, G. T., Console, G. M., Podhajcer, O., Reggiani, P. C., & Goya, R. G. (2019). Mesenchymal stem cell therapy improves spatial memory and hippocampal structure in aging rats. Behavioural Brain Research, 374, 111887. https://doi.org/10.1016/j.bbr.2019.04.001.
Lehmann, M., Zappa-Villar, M. F., García, M. G., Mazzolini, G., Canatelli-Mallat, M., Morel, G. R., Reggiani, P. C., & Goya, R. G. (2019). Umbilical cord cell therapy improves spatial memory in aging rats. Stem Cell Reviews, 22, 612–617. https://doi.org/10.1007/s12015-019-09895-2 [Epub ahead of print].
Bayo, J., Fiore, E., Aquino, J. B., Malvicini, M., Rizzo, M., Peixoto, E., Andriani, O., Alaniz, L., Piccioni, F., Bolontrade, M., Podhajcer, O., Garcia, M. G., & Mazzolini, G. (2014). Increased migration of human mesenchymal stromal cells by autocrine motility factor (AMF) resulted in enhanced recruitment towards hepatocellular carcinoma. PLoS One, 9(4), e95171. https://doi.org/10.1371/journal.pone.0095171.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D., & Horwitz, E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8, 315–317. https://doi.org/10.1080/14653240600855905.
Aquino, J. B., Bolontrade, M. F., García, M. G., Podhajcer, O. L., & Mazzolini, G. (2010). Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Therapy, 17, 692–708. https://doi.org/10.1038/gt.2010.10.
Nishida, F., Zanuzzi, C. N., Márquez, M., Barbeito, C. G., & Portiansky, E. L. (2014). La trepanación vertebral como un método alternativo para la inoculación intraparenquimatosa de diversas suspensiones dentro de la médula espinal. Analecta Veterinaria, 34, 11–17.
Paxinos, G., & Watson, C. (1998). The rat brain in stereotaxic coordinates (4th ed.). Academic: San Diego.
Milano, J., Oliveira, S. M., Rossato, M. F., Sauzem, P. D., Machado, P., Beck, P., Zanatta, N., Martins, M. A., Mello, C. F., Rubin, M. A., Ferreira, J., & Bonacorso, H. G. (2008). Antinociceptive effect of novel trihalomethyl-substituted pyrazoline methyl esters in formalin and hot-plate tests in mice. European Journal of Pharmacology, 581, 86–96. https://doi.org/10.1016/j.ejphar.2007.11.042.
Nishida, F., Morel, G. R., Hereñú, C. B., Schwerdt, J. I., Goya, R. G., & Portiansky, E. L. (2011). Restorative effect of intracerebroventricular Insulin-Like Growth Factor-I gene therapy on motor performance in aging rats. Neuroscience, 177, 195–206. https://doi.org/10.1016/j.neuroscience.2011.01.013.
Metz, G. A., & Whishaw, I. Q. (2002). Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: A new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. Journal of Neuroscience Methods, 115, 169–179.
Portiansky, E. L. (2018). Análisis Multidimensional de Imágenes Digitales (2a Ed.). La Plata: Universidad Nacional de La Plata. Formato digital PDF. ISBN:978-950-34-1713-3. http://sedici.unlp.edu.ar/handle/10915/70938
Kean, T. J., Lin, P., Caplan, A. L., & Dennis, J. E. (2013). MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells International, 2013, 732742. https://doi.org/10.1155/2013/732742.
Huang, P., Freeman, W. D., Edenfield, B. H., Brott, T. G., Meschia, J. F., & Zubair, A. C. (2019). Safety and efficacy of intraventricular delivery of bone marrow-derived mesenchymal stem cells in hemorrhagic stroke model. Scientific Reports, 9, 5674. https://doi.org/10.1038/s41598-019-42182-1.
Gutova, M., Flores, L., Adhikarla, V., Tsaturyan, L., Tirughana, R., Aramburo, S., Metz, M., Gonzaga, J., Annala, A., Synold, T. W., Portnow, J., Rockne, R. C., & Aboody, K. S. (2019). Quantitative evaluation of intraventricular delivery of therapeutic neural stem cells to orthotopic glioma. Frontiers in Oncology, 9, 68. https://doi.org/10.3389/fonc.2019.00068.
Chua, S. J., Bielecki, R., Yamanaka, N., Fehlings, M. G., Rogers, I. M., & Casper, R. F. (2010). The effect of umbilical cord blood cells on outcomes after experimental traumatic spinal cord injury. Spine, 35, 1520–1526. https://doi.org/10.1097/BRS.0b013e3181c3e963.
Kao, C. H., Chen, S. H., Chio, C. C., & Lin, M. T. (2008). Human umbilical cord blood-derived CD34 cells may attenuate spinal cord injury by stimulating vascular endothelial and neurotrophic factors. Shock, 29, 49–55. https://doi.org/10.1097/shk.0b013e31805cddce.
Ryan, J. M., Barry, F. P., Murphy, J. M., & Mahon, B. P. (2005). Mesenchymal stem cells avoid allogeneic rejection. Journal of Inflammation (London), 2, 8. https://doi.org/10.1186/1476-9255-2-8.
Gaudet, A. D., & Fonken, L. K. (2018). Glial cells shape pathology and repair after spinal cord injury. Brain, Behavior, and Immunity, 73, 133–148. https://doi.org/10.1016/j.bbi.2018.07.012.
Lukovic, D., Stojkovic, M., Moreno-Manzano, V., Jendelova, P., Sykova, E., Bhattacharya, S. S., & Erceg, S. (2015). Concise review: Reactive astrocytes and stem cells in spinal cord injury: Good guys or bad guys? Stem Cells, 33(4), 1036–1041. https://doi.org/10.1002/stem.
Krupa, P., Vackova, I., Ruzicka, J., Zaviskova, K., Dubisova, J., Koci, Z., Turnovcova, K., Urdzikova, L. M., Kubinova, S., Rehak, S., & Jendelova, P. (2018). The effect of human mesenchymal stem cells derived from wharton’s jelly in spinal cord injury treatment is dose-dependent and can be facilitated by repeated application. International Journal of Molecular Sciences, 19(5), E1503. https://doi.org/10.3390/ijms19051503.
Chen, C., Chen, F., Yao, C., Shu, S., Feng, J., Hu, X., Hai, Q., Yao, S., & Chen, X. (2016). Intrathecal injection of human umbilical cord-derived mesenchymal stem cells ameliorates neuropathic pain in rat. Neurochemical Research, 41, 3250–3260. https://doi.org/10.1007/s11064-016-2051-5.
Salgado, A. J., Fraga, J. S., Mesquita, A. R., Neves, N. M., Reis, R. L., & Sousa, N. (2010). Role of human umbilical cord mesenchymal progenitors conditioned media in neuronal/glial cell densities, viability, and proliferation. Stem Cells and Development, 19, 1067–1074. https://doi.org/10.1089/scd.2009.0279.
Abbaszadeh, H. A., Tiraihi, T., Noori-Zadeh, A., Delshad, A. R., Sadeghizade, M., & Taheri, T. (2015). Human ciliary neurotrophic factor-overexpressing stable bone marrow stromal cells in the treatment of a rat model of traumatic spinal cord injury. Cytotherapy, 17, 912–921. https://doi.org/10.1016/j.jcyt.2015.03.689.
Hofstetter, C. P., Schwarz, E. J., Hess, D., Widenfalk, J., El Manira, A., Prockop, D. J., & Olson, L. (2002). Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proceedings of the National Academy of Sciences of the United States of America, 99, 2199–2204. https://doi.org/10.1073/pnas.042678299.
Ohta, M., Suzuki, Y., Noda, T., Ejiri, Y., Dezawa, M., Kataoka, K., Chou, H., Ishikawa, N., Matsumoto, N., Iwashita, Y., Mizuta, E., Kuno, S., & Ide, C. (2004). Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Experimental Neurology, 187, 266–278. https://doi.org/10.1016/j.expneurol.2004.01.021.
Falkner, S., Grade, S., Dimou, L., Conzelmann, K. K., Bonhoeffer, T., Götz, M., Hübener, M., Wuttke, T. V., Markopoulos, F., Padmanabhan, H., & Wheeler, A. P. (2016). Transplanted embryonic neurons integrate into adult neocortical circuits. Nature, 539, 248–253. https://doi.org/10.1038/nature20113.
Wuttke, T. V., Markopoulos, F., Padmanabhan, H., Wheeler, A. P., Murthy, V. N., & Macklis, J. D. (2018). Developmentally primed cortical neurons maintain fidelity of differentiation and establish appropriate functional connectivity after transplantation. Nature Neuroscience, 21, 517–529. https://doi.org/10.1038/s41593-018-0098-0.
Abrams, M. B., Dominguez, C., Pernold, K., Reger, R., Wiesenfeld-Hallin, Z., Olson, L., & Prockop, D. (2009). Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restorative Neurology and Neuroscience, 27, 307–321. https://doi.org/10.3233/RNN-2009-0480.
Kang, S. K., Shin, M. J., Jung, J. S., Kim, Y. G., & Kim, C. H. (2006). Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells and Development, 15, 583–594. https://doi.org/10.1089/scd.2006.15.583.
Lin, W., Xu, L., Zwingenberger, S., Gibon, E., Goodman, S. B., & Li, G. (2017). Mesenchymal stem cells homing to improve bone healing. Journal of Orthopaedic Translation, 9, 19–27. https://doi.org/10.1016/j.jot.2017.03.002.
Drommelschmidt, K., Serdar, M., Bendix, I., Herz, J., Bertling, F., Prager, S., Keller, M., Ludwig, A.-K., Duhan, V., Radtke, S., de Miroschedji, K., Horn, P. A., van de Looji, Y., Giebel, B., & Felderhoff-Müser, U. (2017). Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain, Behavior and Immunity, 60, 220–232. https://doi.org/10.1016/j.bbi.2016.11.011.
Kwon, M. S., Noh, M. Y., Oh, K. W., Cho, K. A., Kang, B. Y., Kim, K. S., Kim, Y. S., & Kim, S. H. (2014). The immunomodulatory effects of human mesenchymal stem cells on peripheral blood mononuclear cells in ALS patients. The Journal of Neurochemistry, 131, 206–218. https://doi.org/10.1111/jnc.12814.
Sheikh, A. M., Nagai, A., Wakabayashi, K., Narantuya, D., Kobayashi, S., Yamaguchi, S., & Kim, S. U. (2011). Mesenchymal stem cell transplantation modulates neuroinflammation in focal cerebral ischemia: Contribution of fractalkine and IL-5. Neurobiology of Disease, 41, 717–724. https://doi.org/10.1016/j.nbd.2010.12.009.
Yan, K., Zhang, R., Sun, C., Chen, L., Li, P., Liu, Y., Peng, L., Sun, H., Qin, K., Chen, F., Lv, B., Du, M., Zou, Y., Cai, Y., Qin, L., Tang, Y., & Jiang, X. (2013). Bone marrow-derived mesenchymal stem cells maintain the resting phenotype of microglia and inhibit microglial activation. PLoS One, 8, e84116. https://doi.org/10.1371/journal.pone.0084116.
Wang, L., Pei, S., Han, L., Guo, B., Li, Y., Duan, R., Yao, Y., Xue, B., Chen, X., & Jia, Y. (2018). Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFκB P65 subunit in spinal cord injury. Cellular Physiology and Biochemistry, 50(4), 1535–1559. https://doi.org/10.1159/000494652.
Kim, C., Kim, H. J., Lee, H., Lee, H., Lee, S. J., Lee, S. T., Yang, S. R., & Chung, C. K. (2019). Mesenchymal stem cell transplantation promotes functional recovery through MMP2/STAT3 related astrogliosis after spinal cord injury. International Journal of Stem Cells, 12(2), 331–339. https://doi.org/10.15283/ijsc18133.
Zamanian, J. L., Xu, L., Foo, L. C., Nouri, N., Zhou, L., Giffard, R. G., & Barres, B. A. (2012). Genomic analysis of reactive astrogliosis. Journal of Neuroscience, 32, 6391–6410. https://doi.org/10.1523/JNEUROSCI.6221-11.2012.
Liddelow, S. A., Guttenplan, K. A., Clarke, L. E., Bennett, F. C., Bohlen, C. J., Schirmer, L., Bennett, M. L., Münch, A. E., Chung, W. S., Peterson, T. C., Wilton, D. K., Frouin, A., Napier, B. A., Panicker, N., Kumar, M., Buckwalter, M. S., Rowitch, D. H., Dawson, V. L., Dawson, T. M., Stevens, B., & Barres, B. A. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. https://doi.org/10.1038/nature21029.
Liu, W., Wang, Y., Gong, F., Rong, Y., Luo, Y., Tang, P., Zhou, Z., Zhou, Z., Xu, T., Jiang, T., Yang, S., Yin, G., Chen, J., Fan, J., & Cai, W. (2019). Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of a1 neurotoxic reactive astrocytes. Journal of Neurotrauma, 36(3), 469–484. https://doi.org/10.1089/neu.2018.5835.
Zappa Villar, M. F., López Hanotte, J., Pardo, J., Morel, G. R., Mazzolini, G., García, M. G., & Reggiani, P. C. (2019). Mesenchymal stem cells therapy improved the streptozotocin-induced behavioral and hippocampal impairment in rats. Molecular Neurobiology, 1–16. https://doi.org/10.1007/s12035-019-01729-z [Epub ahead of print].
Wilhelmsson, U., Faiz, M., de Pablo, Y., Sjöqvist, M., Andersson, D., Widestrand, A., Potokar, M., Stenovec, M., Smith, P. L., Shinjyo, N., Pekny, T., Zorec, R., Ståhlberg, A., Pekna, M., Sahlgren, C., & Pekny, M. (2012). Astrocytes negatively regulate neurogenesis through the Jagged 1-mediated Notch pathway. Stem Cells, 30, 2320–2329. https://doi.org/10.1002/stem.1196.
Shi, W., Huang, C. J., Xu, X. D., Jin, G. H., Huang, R. Q., Huang, J. F., Chen, Y. N., Ju, S. Q., Wang, Y., Shi, Y. W., Qin, J. B., Zhang, Y. Q., Liu, Q. Q., Wang, X. B., Zhang, X. H., & Chen, J. (2016). Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomaterialia, 45, 247–261. https://doi.org/10.1016/j.actbio.2016.09.001.
Huang, J. H., Yin, X. M., Xu, Y., Xu, C. C., Lin, X., Ye, F. B., Cao, Y., & Lin, F. Y. (2017). Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. Journal of Neurotrauma, 34(24), 3388–3396. https://doi.org/10.1089/neu.2017.5063.
Wrathall, J. R., Teng, Y. D., & Marriott, R. (1997). Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Experimental Neurology, 145(2 Pt 1), 565–573.
Acknowledgements
This work was supported by the National Agency for Promotion of Science and Technology (ANPCyT) (grant PICT 2015-2087 to FN and PICT 2015-1998 to PCR) and by the National University of La Plata (grant V270 to ELP). The authors thank Dr. G. Mazzolini and Dr. M.G. García (Universidad Austral, CONICET) for kindly providing the hUC-MSC. The expert handling of animals by Mr. H. Enrique is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors do not have any financial conflict of interests to disclose.
Research Involving Animals
All experiments with animals were performed according to the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was also approved by the School of Veterinary Sciences, National University of Plata Institutional Committee for Care and Use of Laboratory Animals (CICUAL), code n° 49-8-15 P.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishida, F., Zappa Villar, M.F., Zanuzzi, C.N. et al. Intracerebroventricular Delivery of Human Umbilical Cord Mesenchymal Stem Cells as a Promising Therapy for Repairing the Spinal Cord Injury Induced by Kainic Acid. Stem Cell Rev and Rep 16, 167–180 (2020). https://doi.org/10.1007/s12015-019-09934-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09934-y