Abstract
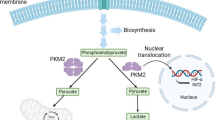
The prolyl hydroxylase 3 (PHD3) protein is less abundant in normal oxygen conditions (normoxia) but increases under deficient oxygen condition (hypoxia). Since cancerous cells often thrive in hypoxic conditions and predominantly express the Pyruvate kinase isoforms 2 (PKM2), the PHD3/PKM2 interaction might be particularly important in cancer development. In the present study, the PHD3/PKM2 complex was co-expressed and purified by size-exclusion chromatography. The interaction of PHD3 with PKM2 was confirmed in Native gel as well as western blot analysis. The PHD3/PKM2 complex formed discreet crystals under suitable conditions, and diffraction data revealed that crystal belonged to the P1 space group with 3.0 Å resolution. This is the first crystal report of PHD3/PKM2 complex as well as this study demonstrates a direct physical binding through protein–protein interaction. The structural analysis of complex will provide the information regarding the amino acid residues critical for the catalytic mechanism. Based on the structural information thus obtained, pharmacological interference with the PHD3/PKM2 interaction could be used as a novel strategy to reduce the cancer progression.






Similar content being viewed by others
References
Hitosugi, T., Kang, S., Vander Heiden, M. G., Chung, T. W., Elf, S., Lythgoe, K., et al. (2009). Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Science Signaling,2, ra73–ra73.
Gui, D. Y., Lewis, C. A., & Vander Heiden, M. G. (2013). Allosteric regulation of PKM2 allows cellular adaptation to different physiological states. Science Signaling,6, pe7–pe7.
Srivastava, D., Razzaghi, M., Henzl, M. T., & Dey, M. (2017). Structural investigation of a dimeric variant of pyruvate kinase muscle isoform 2. Biochemistry,56, 6517–6520.
Luo, W., Hu, H., Chang, R., Zhong, J., Knabel, M., O’Meally, R., et al. (2011). Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell,145, 732–744.
Lee, J., Kim, H. K., Han, Y.-M., & Kim, J. (2008). Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. The International Journal of Biochemistry and Cell Biology,40, 1043–1054.
Yang, W., Xia, Y., Ji, H., Zheng, Y., Liang, J., Huang, W., et al. (2011). Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature,480, 118.
Azoitei, N., Becher, A., Steinestel, K., Rouhi, A., Diepold, K., Genze, F., et al. (2016). PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Molecular Cancer,15, 3.
Chen, N., Rinner, O., Czernik, D., Nytko, K. J., Zheng, D., Stiehl, D. P., et al. (2011). The oxygen sensor PHD3 limits glycolysis under hypoxia via direct binding to pyruvate kinase. Cell Research,21, 983.
Epstein, A. C., Gleadle, J. M., McNeill, L. A., Hewitson, K. S., O’Rourke, J., Mole, D. R., et al. (2001). C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell,107, 43–54.
McNeill, L. A., Flashman, E., Buck, M. R., Hewitson, K. S., Clifton, I. J., Jeschke, G., et al. (2005). Hypoxia-inducible factor prolyl hydroxylase 2 has a high affinity for ferrous iron and 2-oxoglutarate. Molecular BioSystems,1, 321–324.
Kaelin, W. G., Jr., & Ratcliffe, P. J. (2008). Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Molecular Cell,30, 393–402.
Semenza, G. L. (2010). Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene,29, 625.
Hasan, D., Gamen, E., Tarboush, N. A., Ismail, Y., Pak, O., & Azab, B. (2018). PKM2 and HIF-1α regulation in prostate cancer cell lines. PLoS ONE,13, e0203745.
Metzen, E., Berchner-Pfannschmidt, U., Stengel, P., Marxsen, J. H., Stolze, I., Klinger, M., et al. (2003). Intracellular localisation of human HIF-1α hydroxylases: Implications for oxygen sensing. Journal of Cell Science,116, 1319–1326.
Christofk, H. R., Vander Heiden, M. G., Harris, M. H., Ramanathan, A., Gerszten, R. E., Wei, R., et al. (2008). The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature,452, 230.
Studier, F. W. (2014). Stable expression clones and auto-induction for protein production in E. coli, in structural genomics (pp. 17–32). Totowa: Humana Press.
Kumar, S., Dhembla, C., Hariprasad, P., Sundd, M., & Patel, A. K. (2019). Differential expression of structural and functional proteins during bean common mosaic virus-host plant interaction. Microbial Pathogenesis. https://doi.org/10.1016/j.micpath.2019.103812.
Kumar, S., Karmakar, R., Garg, D. K., Gupta, I., & Patel, A. K. (2019). Elucidating the functional aspects of different domains of bean common mosaic virus coat protein. Virus Research,273, 197755.
Merril, C. R., Goldman, D., Sedman, S. A., & Ebert, M. H. (1981). Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science,211, 1437–1438.
Kabsch, W. (2010). XDS. Acta Crystallographica Section D,66, 125–132.
Winn, M. D., Ballard, C. C., Cowtan, K. D., Dodson, E. J., Emsley, P., Evans, P. R., et al. (2011). Overview of the CCP4 suite and current developments. Acta Crystallographica Section D,67, 235–242.
Roy, A., Kucukural, A., & Zhang, Y. (2010). I-TASSER: A unified platform for automated protein structure and function prediction. Nature Protocols,5, 725.
McDonough, M. A., Li, V., Flashman, E., Chowdhury, R., Mohr, C., Liénard, B. M., et al. (2006). Cellular oxygen sensing: CRYSTAL structure of hypoxia-inducible factor prolyl hydroxylase (PHD2). Proceedings of the National Academy of Sciences,103, 9814–9819.
Laskowski, R. A., MacArthur, M. W., Moss, D. S., & Thornton, J. M. (1993). PROCHECK: A program to check the stereochemical quality of protein structures. Journal of Applied Crystallography,26, 283–291.
Yan, Y., Zhang, D., Zhou, P., Li, B., & Huang, S.-Y. (2017). HDOCK: A web server for protein–protein and protein–DNA/RNA docking based on a hybrid strategy. Nucleic Acids Research,45, W365–W373.
Matsui, Y., Yasumatsu, I., Asahi, T., Kitamura, T., Kanai, K., Ubukata, O., et al. (2017). Discovery and structure-guided fragment-linking of 4-(2, 3-dichlorobenzoyl)-1-methyl-pyrrole-2-carboxamide as a pyruvate kinase M2 activator. Bioorganic and Medicinal Chemistry,25, 3540–3546.
Fedulova, N., Hanrieder, J., Bergquist, J., & Emrén, L. O. (2007). Expression and purification of catalytically active human PHD3 in Escherichia coli. Protein Expression and Purification,54, 1–10.
Yang, W. (2015). Structural basis of PKM2 regulation. Protein and Cell,6, 238–240.
Acknowledgements
We are thankful to the beamline scientists at the European Synchrotron Radiation Facility, Grenoble, France for assisting us with the use of beamline ID30A-3. The authors acknowledge the infrastructural support from Indian Institute of Technology Delhi. SK acknowledges the research grant from SERB, Department of Science & Technology, and Govt. of India. Authors thank Dr. Dushyant Garg and Dr. Ruma Karmakar for continuous help in research experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, S., Patel, A.K. Purification and Characterization of Prolyl Hydroxylase 3/Pyruvate Kinase Isoform 2 Protein Complex. Mol Biotechnol 62, 111–118 (2020). https://doi.org/10.1007/s12033-019-00228-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-019-00228-9