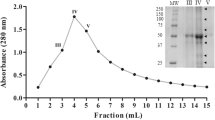
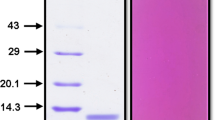
Abstract
Protease inhibitors are crucial for the control of proteolytic activity in different physiological processes. However, some inhibitors do not show canonical enzyme recognition of the enzyme under certain conditions. In this work, we present evidence that indicates the formation of an active complex between the protease bovine α-chymotrypsin and the Tepary bean protease inhibitor (TBPI). The composition of the active chymotrypsin-TBPI complex (AC) was confirmed by three different methods: size-exclusion chromatography, polyacrylamide gel electrophoresis (PAGE), and mass spectrometry. The kinetic parameters for the AC were similar to those of the enzyme alone, indicating that TBPI binding does not produce any large changes in chymotrypsin. The molecular model proposed here postulates that TBPI binds outside the active cleft of the protease, but near enough to hinder the binding of high molecular weight substrates into the active site. This model was experimentally supported by the inhibitory effect on casein as a substrate, and the unaltered protease activity when a small synthetic substrate was used. We also found that the formation of this complex provided the enzyme with extra stability in denaturing conditions or in the presence of a reducing agent. The chymotrypsin-TBPI complex exhibited higher stability, indicating that autolysis can be partially prevented. When the enzyme was first inactivated followed by the addition of the inhibitor, the activity of the protease was restored. We described a possible mechanism where a plant protease inhibitor binds outside the active site of the enzyme while increasing its stability.










Similar content being viewed by others
Abbreviations
- AC:
-
Active chymotrypsin-TBPI complex
- appMM:
-
Apparent molecular mass
- AU:
-
Proteolytic activity units
- BApNA:
-
N-benzoyl-arginine-p-nitroanilide
- BBI:
-
Bowman-Birk type inhibitor
- BSA:
-
Bovine serum albumin
- Da:
-
Daltons
- DMSO:
-
Dimethyl sulfoxide
- DTT:
-
Dithiothreitol
- E:
-
Enzyme/α-chymotrypsin
- EI:
-
Enzyme-Inhibitor/chymotrypsin-TBPI
- IC:
-
Inactive chymotrypsin-TBPI complex
- IU:
-
Inhibition activity units
- Km:
-
Michaelis–Menten constant
- M:
-
Molar
- MALDI-TOF-TOF:
-
Matrix assisted laser desorption ionization time-of-flight
- MMP:
-
Matrix metalloproteinase
- MS/MS:
-
Tandem mass spectrometry
- PAGE:
-
Polyacrylamide gel electrophoresis
- PI:
-
Protease inhibitor
- PI’s:
-
Protease inhibitors
- SAAFpNA:
-
N-Succinyl-Ala-Ala-Pro-Phe-p-nitroanilide
- SDS:
-
Sodium dodecyl sulfate
- SDS-PAGE:
-
Polyacrylamide gel electrophoresis with sodium dodecyl sulfate
- TBPI:
-
Tepary bean protease inhibitor
- TEMED:
-
1,2-bis(dimethylamine)-ethane
- Tm:
-
Melting temperature
- Vmax:
-
Maximum velocity
References
Habib H, Fazili MK (2007) Plant protease inhibitors: a defense strategy in plants. Biotechnol Mol Biol Rev 2:68–85
Page MJ, Di Cera E (2008) Serine peptidases: classification, structure and function. Cell Mol Life Sci 65:1220–1236
Di Cera E (2009) Serine proteases. IUBMB Life 61:510–515
Khan AR, James MNG (1998) Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci 7:815–836
Krowarsch D, Cierpicki T, Jelen F, Otlewski J (2003) Canonical protein inhibitors of serine proteases. Cell Mol Life Sci 60:2427–2444
Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ (2008) MEROPS: the peptidase database. Nucleic Acids Res 36:320–325
Ohlsson K, Skude G (1976) Demonstration and semiquantitative determination of complexes between various proteases and human α2- macroglobulin. Clin Chim Acta 66:1–7
Gliemann J, Sottrup-Jensen L (1987) Rat plasma α1-inhibitor3 binds to receptors for α2-macroglobulin. FEBS Lett 221:55–60
Gauthier F, Genell S, Mouray H, Ohlsson K (1979) Interactions in vitro and in vivo between rat serum protease inhibitors and anodal and cathodal rat trypsin and chymotrypsin. Biochim Biophys Acta 566:200–210
Esnard F, Gutman N, El Moujahed A, Gauthier F (1985) Rat plasma α1- inhibitor3: a member of the α-macroglobulin family. FEBS Lett 182:125–129
Qiu WQ, Borth W, Ye Z, Haass C, Teplow DB, Selkoe DJ (1995) Degradation of amyloid-protein by a serine protease α-macroglobulin complex. J Biol Chem 271:8443–8451
Tchetverikov I, Lard LR, DeGroot J, Verzijl N, TeKoppele JM, Breedveld FC, Huizinga TWJ, Hanemaaijer R (2003) Matrix metalloproteinases-3, -8, -9 as markers of disease activity and joint damage progression in early rheumatoid arthritis. Ann Rheum Dis 62:1094–1099
Ray S, Lukyanov P, Ochieng J (2003) Members of the cystatin superfamily interact with MMP-9 and protect it from autolytic degradation without affecting its gelatinolytic activities. Biochim Biophys Acta 1652:91–102
Mukherjee AK, Sumita D, Bhargab K, Deepak KJ, Pritam D, Stephen PM (2016) Structural and functional characterization of complex formation between two Kunitz-type serine protease inhibitors from Russell’s Viper venom. Biochimie 138:128–129
Guilloteau M, Laloi M, Michaux S, Bucheli P, McCarthy J (2005) Identification and characterization of the major aspartic proteinase activity in Theobroma cacao seeds. J Sci Food Agric 85:549–562
Castro-Guillén JL (2012) Caracterización parcial de serinpeptidasas de Prostephanus truncates. Dissertation CINVESTAV, Unidad Irapuato, México
Molnár T, Vörös J, Szeder B, Takáts K, Kardos J, Katona G, Gráf L (2013) Comparison of complexes formed by a crustacean and a vertebrate trypsin with bovine pancreatic trypsin inhibitor—the key to achieving extreme stability? FEBS Lett 280:5750–5763
Campos JE, Martinez-Gallardo N, Mendiola-Olaya E, Blanco-Labra A (1997) Purification and partial characterization of a proteinase inhibitor from Tepary bean (Phaseolus acutifolius seeds). J Food Biochem 21:203–218
Campos JE, Whitaker RJ, Yip TT, Hutchens WT, Blanco-Labra A (2004) Unusual structural characteristics and complete amino acid sequence of a protease inhibitor from Phaseolus acutifolius seeds. Plant Physiol Biochem 42:209–214
Schägger H, Von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Erlanger B, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95:271–278
Fernández-Resa P, Mira E, Quesada AR (1995) Enhanced detection of casein zymography of matrix metalloproteinase. Anal Biochem 224:434–435
Vinokurov KS, Oppert B, Elpidina EN (2005) An overlay technique for post-electrophoretic analysis of proteinase spectra in complex mixtures using p-nitroanilide substrates. Anal Biochem 337:164–166
Ohlsson BG, Weström BR, Karlsson BW (1986) Enzyme-blotting: a method for localizing proteinases and their zymogens using para-nitroanilide substrates after agarose gel electrophoresis and transfer to nitrocellulose. Anal Biochem 152:239–244
Hellman U, Wernstedt C, Gonez J, Heldin CH (1995) Improvement of an “in-gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal Biochem 224:451–455
Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem 68:850–858
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Schwede T (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42(W1):W252–W258
Zhang J, Liang Y, Zhang Y (2011) Atomic-level protein structure refinement using fragment-guided molecular dynamics conformation sampling. Structure 19(12):1784–1795
Kozakov D, Hall DR, Xia B, Porter KA, Padhorny D, Yueh C, Vajda S (2017) The ClusPro web server for protein–protein docking. Nat Protoc 12(2):255
Huynh K, Partch CL (2015) Analysis of protein stability and ligand interactions by thermal shift assay. Curr Protoc Protein Sci 29:21–34
Chen P, Rose J, Love R, Wei CH, Wang BC (1992) Reactive sites of an anticarcinogenic Bowman-Birk proteinase inhibitor are similar to other trypsin inhibitors. J Biol Chem 267(3):1990–1994
Kumar P, Rao AA, Hariharaputran S, Chandra N, Gowda LR (2004) Molecular Mechanism of Dimerization of Bowman-Birk Inhibitors pivotal role of asp76 in the dimerzation. J Biol Chem 279(29):30425–30432
Silva LP, Azevedo RB, Morais PC, Ventura MM, Freitas SM (2005) Oligomerization states of Bowman-Birk inhibitor by atomic force microscopy and computational approaches. Proteins 61(3):642–648
Rao KN, Suresh CG (2007) Bowman-Birk protease inhibitor from the seeds of Vigna unguiculata forms a highly stable dimeric structure. Biochem Biophys Acta 1774(10):1264–1273
Fucikova J, Kasikova L, Truxova I, Laco J, Skapa P, Ryska A, Spisek R (2018) Relevance of the chaperone-like protein calreticulin for the biological behavior and clinical outcome of cancer. Immunol Lett 193:25–34
Acknowledgements
We thank CONACYT grant 182760ABL, and fellowships of R Pliego-Arreaga, O Roldán-Padrón and M Dagio-Hernández. To Alicia Chagolla for her efficient assistance with mass spectrometry. Thanks to Dr. Jorge A. Torres-Castillo, and also thanks to Dr. Collen Beard and Carolyn Smith of Peace Corps Response for their useful advice when reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pliego-Arreaga, R., Roldán-Padrón, O., Castro-Guillén, J.L. et al. Properties of a Non-canonical Complex Formed Between a Tepary Bean (Phaseolus acutifolius) Protease Inhibitor and α-Chymotrypsin. Protein J 38, 435–446 (2019). https://doi.org/10.1007/s10930-019-09863-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-019-09863-2