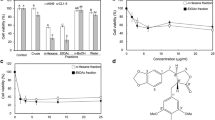
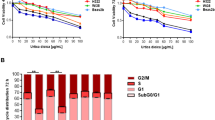
Abstract
The emergence of multi drug resistance in non-small cell lung cancer (NSCLC) patients is a major challenge towards the efficacy of chemotherapy. Thus, there is an urgent need for the newer, better clinically targeted strategies to treat this disease. Earlier studies from our laboratory revealed the apoptotic activity of Maackia amurensis agglutinin (MAA) in human NSCLC cells. In this study, the effect of MAA on drug resistant NSCLC cells was investigated. Two Paclitaxel-resistant NSCLC sub-lines (A549/PTX100 and NCI-H460/PTX100) were developed from A549 & NCI-H460 cell lines respectively. The generation of drug resistance phenotype was confirmed by the expression of cell surface MDR-1. Both the drug resistant sub-lines showed distinct morphological alterations. MAA interacted with the cell-surface protein(s) of apparent Mr ~66 kDa and induced apoptosis in both the sub-lines through intrinsic/mitochondrial pathway, involving reduction in mitochondrial trans-membrane potential, up-regulation of Bax, unaltered/decreased expression of Bcl-XL, release of mitochondrial cytochrome c into the cytosol and activation of pro-caspases (−9&-3). Our findings highlighted the potential of this plant agglutinin to serve as an apoptosis inducing agent in drug resistant NSCLC cells.






Similar content being viewed by others
References
Didkowska, J., Wojciechowska, U., Manczuk, M., Lobaszewski, J.: Lung Cancer epidemiology : contemporary and future challenges worldwide. Ann Transl Med. 4, 15–161 (2016)
American Cancer Society (2017) Cancer Facts and Figures
Joshi, M., Liu, X., Belani, C.P.: Taxanes, past, present and future impact on non-small cell lung cancer. Anti-Cancer Drugs. 25, 571–583 (2014)
Szakacs, G., Paterson, J.K., Ludwig, J.A., Booth-Genthe, C., Gottesman, M.M.: Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5, 219–234 (2006)
Eid, S.Y., El-Readi, M.Z., Fatani, S.H., Eldin, E.E.M.N., Wink, M.: Natural products modulate the multifactorial multidrug resistance of cancer. Pharmacol. Pharm. 6, 146–176 (2015)
Shanker, M., Willcutts, D., Roth, J.A., Ramesh, R.: Drug resistance in lung cancer. Lung Cancer Target. Ther. 1, 23–36 (2010)
Zahreddine, H., Borden, K.L.B.: Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 4, 1–8 (2013)
Yakymovych, M.Y., Yakymovych, I.A., Chekhun, V.F., Stoika, R.S.: Different production of TGF-β1 by cisplatin-sensitive and resistance L1210 cells treated with anticancer drug and lectins. Exp. Oncol. 23, 204–208 (2001)
Valentiner, U., Pfuller, U., Baum, C., Schumacher, U.: The cytotoxic effect of mistletoe lectins I, II and III on sensitive and multidrug resistant human colon cancer cell lines in vitro. Toxicology. 17, 187–199 (2002)
Beztsinna, N., de Matos, M.B.C., Walther, J., Heyder, C., Hildebrandt, E., Leneweit, G., Mastrobattista, E., andKok, R.J.: Analysis of receptormediated uptake and pro-apoptotic activity of mistletoe lectin-1 by high content imaging. Sci. Rep. 8, 2768 (2018). https://doi.org/10.1038/s41598-018-20915-y
Chen, K., Yang, X., Wu, L., Yu, M., L, X., Li, N., Wang, S., Li, G.: Pinelliapedatisecta agglutinin targets drug resistant K562/ADR leukemia cells through binding with sarcolemmal membrane associated protein and enhancing macrophage phagocytosis. PLoS One. 8(9), e74363 (2013). https://doi.org/10.1371/journal.pone.0074363
Fedoreev, S.A., Kulish, N.I., Glebko, L.I., Pokushalova, T.V., Veselova, M.V., Saratikov, A.S., Vengerovskii, A.I., Chuchalin, V.S.: Maksar: A preparation based on Amur Maackia. Pharmaceutical Chem. J. 38, 1–7 (2004)
Kawaguchi, T., Matsumoto, I., Osawa, T.: Studies on hemagglutinins from Maackia amurensis seeds. J. Biol. Chem. 10, 2786–2792 (1974)
Wang, W.-C., Cummings, R.D.: As assay for leukoagglutinating lectins using suspension cultured mouse lymphoma cells (BW5147) stained with neutral red. Anal. Biochem. 161, 80–84 (1987)
Ohyama, C., Hosono, M., Nitta, K., Oh-eda, M., Yoshikawa, K., Habuchi, T., Arai, Y., Fukuda, M.: Carbohydrate structure and differential binding of prostrate specific antigen to Maackia amurensis lectin between prostrate cancer and benign prostrate hypertrophy. Glycobiology. 14, 671–679 (2004)
Tang, W., Guo, Q., Mayumi, U., Norihiko, K., Yasuji, S., Masami, M., Yasuhiko, S., Munehiro, N., Naoya, K., Masatoshi, M.: Histochemical expression of sialoglycoconjugates in carcinoma of the papilla of vater. Hepatogastroenterology. 52, 67–71 (2004)
Babal, P., Janega, P., Cerna, A., Kholova, I.: Neoplastic transformation of the thyroid gland is accompanied by changes in cellular sialylation. Acta Histochemica. 108, 133–140 (2006)
Inagaki, Y., Tang, W., Guo, Q., Kokudo, N., Sugawara, Y., Karako, H., Konishi, T., Nakata, M., Nagawa, H., Makuuchi, M.: Sialoglycoconjugate expression in primary colorectal and metastatic lymph node tissues. Hepatogastroenterology. 54, 53–57 (2007)
Inagaki, Y., Usuda, M., Xu, H., Wang, F., Cui, S., Mafune, K.-L., Sugawara, Y., Kokudo, N., Tang, W., Nakata, M.: Apperance of high molecular weight sialoglycoproteins recognized by Maackia amurensis leukoagglutinin in gastic cancer tissues.: a case report using 2-DE -lectin binding analysis. Biosci. Trends. 2, 151–154 (2008)
Peracaula, R., Barrabes, S., Sarrats, A., Rudd, P.M., de Llorens, R.: Altered glycosylation in tumours focused to cancer diagnosis. Dis. Markers. 25, 207–218 (2008)
Fukasawa, T., Asao, T., Yamauchi, H., Ide, M., Tabe, Y., Fujii, T., Yamaguchi, S., Tsutsumi, S., Yazawa, S., Kuwano, H.: Associated expression of α2,3 sialylated type 2 chain structures with lymph node metastasis in distal colorectal cancer. Surg. Today. 43, 155–162 (2013)
Ochoa-Alvarez, J.A., Krishnan, H., Shen, Y., Acharya, N.K., Han, M., McNulty, D.E., Hasegawa, H., Hyodo, T., Senga, T., Geng, J.G., Kosciuk, M., Shin, S.S., Goydos, J.S., Temiakov, D., Nagele, R.G., Goldberg, G.S.: Plant lectin can target receptors containing sialic acid, exemplified by podoplanin, to inhibit transformed cell growth and migration. PloS One. 7, e41845 (2012)
Kapoor, S., Marwaha, R., Majumdar, S., Ghosh, S.: Apoptosis induction by Maackia amurensis agglutinin in childhood acute lymphoblastic leukemic cells. Leuk. Res. 3, 559–567 (2008)
Mehta, S., Chhetra, R., Srinivasan, R., Sharma, S.C., Behera, D., Ghosh, S.: Potential importance of Maackia amurensis agglutinin in non-small cell lung cancer. Biol. Chem. 394, 889–900 (2013)
Lalli, R.C., Kaur, K., Dadsena, S., Chakraborti, A., Srinivasan, R., Ghosh, S.: Maackiaamurensis agglutinin enhances paclitaxel induced cytotoxicity in cultured non-small cell lung cancer cells. Biochimie. 115, 93–107 (2015)
Yabuki, N., Sakata, K., Yamasaki, T., Terashima, H., Mio, T., Miyazaki, Y., Fujii, T., Kitada, K.: Gene amplification and expression in lung cancer cells with acquired paclitaxel resistance. Cancer Genet. Cytogenet. 173, 1–9 (2007)
Bui, K.C., Buckley, S., Wu, F., Uhal, B., Joshi, I., Liu, J., Hussain, M., Makhoul, I., Warburton, D.: Induction of A-and D type cyclins and cdc2 kinase activity during recovery from short-term hyperoxic lung injury. Am. J. Phys. 268, L625–L635 (1995)
Smith, P.K., Krohn, R.I., Hermanson, G.T., Mallia, A.K., Gartner, F.H., Provenzano, M.D., Fujimoto, E.K., Goeke, N.M., Olson, B.J., Klenk, D.C.: Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85 (1985)
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227, 680–685 (1970)
Towbin, H., Staehelin, T. and Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications, Proc. Natl.Acad.Sci. U S A., 76, 4350–4354
Bui, K.C., Buckley, S., Wu, F., Uhal, B., Joshi, I., Liu, J., Hussain, M., Makhoul, I., Warburton, D.: Induction of A-and D type cyclins and cdc2 kinase activity during recovery from short-term hyperoxic lung injury. Am.J.Physiol. 268, L625–L635 (1985)
Nicoletti, I., Migliorati, G., Pagliacci, M.C., Grignani, F., Riccardi, C.: A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 139, 271–279 (1991)
Fabbri, F., Carloni, S., Brigliadori, G., Zoli, W., Lapalombella, R., andMarini, M.: Sequential events of apoptosis involving docetaxel, a microtubule-interfering agent: a cytometric study. BMC Cell Biol. 7, 1–14 (2006)
Chomczynski, P., Sacchi, N.: Single-step method of RNA isolation by acid guanidiniumthiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 (1987)
Livak, K.J., Schmittgen, T.D.: Analysis of a relative gene expression data using real- time quantitative PCR and the 2-ΔΔC T method. Methods. 25, 402–408 (2001)
Kim, W.H., Park, W.B., Gao, B., Jung, M.H.: Critical role of reactive oxygenspecies and mitochondrialmembrane potential in Korean mistletoe lectin-induced apoptosis in human hepatocarcinoma cells. Mol. Pharmacol. 66, 1383–1396 (2004)
Wang, J., Seebacher, N., Shi, H., Kan, Q., Duan, Z.: Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget. 8(48), 84559–84571 (2017)
Sun, Q.L., Sha, H.F., Yang, X.H., Bao, G.L., Lu, J., Xie, Y.Y.: Comparative proteomic analysis of paclitaxel sensitive A549 lung adenocarcinoma cell line and its resistant counterpart A549-Taxol. J. Cancer Res. Clin. Oncol. 137, 521–532 (2011)
Uhrik, B., El-Saggan, A.H., Seres, M., Gibalova,L., Breier, A., Sulova, Z. (2006) Structural differences between sensitive and resistant L1210 cells, Gen. Physiol. Biophys. 25427–25438
Zhao, R., Qin, W., Qin, R., Han, J., Li, C., Wang, Y., Xu, C.: Lectin array andglycogene expression analyses of ovarian cancer cell line A2780 and its cisplatin-resistant derivate cell line A2780-cp. Clin. Proteomics. 14, 20 (2017). https://doi.org/10.1186/s12014-017-9155-z
Hu, X., Christian, P., Sipes, I.G., Hoyer, P.B.: Expression and redistribution of cellular Bad, Bax, and Bcl-X protein is asssociatd with VCD induced ovotoxicity in rats. 65, 1489-1495 (2001).
Asmarinah, A., Paradowska-Dogan, A., Kodariah, R., Tanuhardja, B., Waliszewski, P., Mochtar, C.A., Budiningsih, Y., Weidner, W., Hinsch, E.: Expression of the Bcl-2 family genes and complexes involved in the mitochondrial transport in prostate cancer cells. Int. J. Oncol. 45, 1489–1496 (2014)
Arumugam, J., Jeddy, N., Ramamurthy, A., Thangavelu, R.: The expression of Bcl-2 in oral squamous cell carcinoma. J. Orofac. Sci. 9, 71–74 (2017)
Lyu, S.Y., Choi, S.H., Park, W.B.: Korean mistletoe lectin-induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch. Pharm. Res. 25, 93–101 (2002)
Hassaan, M., Watari, H., Almaaty, A.A., Ohba, Y., Sakuragi, N.: Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014, 1–23 (2014)
Kong, C.Z., Zhang, Z.: Bcl-2 overexpression inhibits generation of intracellular reactive oxygen species and blocks adriamycin-induced apoptosis in bladder cancer cells. Asian Pac. J. Cancer Prev. 14, 895–901 (2013)
Peng, Y., Wang, L., Qing, Y., Li, C., Ren, T., Li, Q., Li, M., Zhang, S., Shan, J., Wang, G., Yang, Z., Wang, D.: Polymorphisms of Bcl-2 and Bax genes associate with outcomes in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Sci. Rep. 5, 17766 (2015)
Tzifi, F., Economopoulou, C., Gourgiotis, D., Ardavanis, A., Papageorgiou, S., Scorilas, A.: The role of Bcl-2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv. Hematol. 2012, 1–15 (2012)
deGraaff, M.A., de Rooij, M.A., van den Akker, B.E., Gelderblom, H., Chibon, F., Coindre, J.M., Marino-Enriquez, A., Fletcher, J.A., Cleton-Jansen, A.M., Bovee, J.V.: Inhibition of Bcl-2 family members sensitises soft tissue leiomyosarcomas to chemotherapy. Br. J. Cancer. 114, 1219–1226 (2016)
Rao, P.V., Jayaraj, R., Bhaskar, A.S., Kumar, O., Bhattacharya, R., Saxena, P., Dash, P.K., Vijayaraghavan, R.: Mechanism of ricin-induced apoptosis in human cervical cancer cells. Biochem. Pharmacol. 69, 855–865 (2005)
Singh, R., Nawale, L., Sarkar, D., Suresh, C.G.: Two chitotriose-specific lectins show anti-angiogenesis, induces caspase-9-mediated apoptosis and early arrest of pancreatic tumor cell cycle. PLoS One. 11(1), e0146110 (2016). https://doi.org/10.1371/journal.pone.0146110
Tatsuta, T., Hosono, M., Takahashi, K., Omoto, T., Kariya, Y., Sugawara, S., Hakomori, S., Nitta, K.: Sialic acid-binding lectin (leczyme) induces apoptosis to malignant mesothelioma and exerts synergistic antitumor effects with TRAIL. Int. J. Oncol. 44, 377–384 (2014)
Acknowledgements
This work was supported by the grant obtained from Indian Council of Medical Research (New Delhi, India).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 257 kb)
Rights and permissions
About this article
Cite this article
Chhetra Lalli, R., Kaur, K., Chakraborti, A. et al. Maackia amurensis agglutinin induces apoptosis in cultured drug resistant human non-small cell lung cancer cells. Glycoconj J 36, 473–485 (2019). https://doi.org/10.1007/s10719-019-09891-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-019-09891-1