Abstract
Background
The effectiveness of pallidal deep brain stimulation (GPi DBS) for cervical dystonia has been extensively described, but controversies exist about which prognostic factor is clinically useful. We previously reported that classification of tonic- or phasic-type cervical dystonia is useful for predicting clinical prognosis; however, the approach used by physicians to distinguish between the two types remains subjective.
Objective
The aim of this study was to develop a prognostic factor of GPi DBS for cervical dystonia.
Methods
By identifying distributions of range of motion scores between phasic- and tonic-type cervical dystonia, a new prognostic factor group was developed based on whether the patients could voluntarily move their head to the opposite side against dystonic motions. The prognosis for GPi DBS in the two groups was analyzed according to the time sequence.
Results
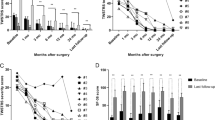
Patients who were able to move their head past the midline had a better long-term prognosis after GPi DBS than did those who could not. In the early post-operative phase, there were no significant differences in the clinical outcomes between the two groups.
Conclusion
A range of voluntary neck motility with respect to the midline is an objective factor that is useful in predicting the prognosis of patients with cervical dystonia. This result renders needs for future study addressing neuroplastic changes in the brain network caused by GPi DBS.



Similar content being viewed by others
References
Altenmuller E, Baur V, Hofmann A, Lim VK, Jabusch HC (2012) Musician’s cramp as manifestation of maladaptive brain plasticity: arguments from instrumental differences. Ann N Y Acad Sci 1252:259–265
Barow E, Neumann WJ, Brucke C, Huebl J, Horn A, Brown P, Krauss JK, Schneider GH, Kuhn AA (2014) Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements. Brain 137:3012–3024
Byl NN, Merzenich MM, Jenkins WM (1996) A primate genesis model of focal dystonia and repetitive strain injury: I. Learning-induced dedifferentiation of the representation of the hand in the primary somatosensory cortex in adult monkeys. Neurology 47:508–520
Calabresi P, Pisani A, Rothwell J, Ghiglieri V, Obeso JA, Picconi B (2016) Hyperkinetic disorders and loss of synaptic downscaling. Nat Neurosci 19:868–875
Chung M, Huh R (2016) Different clinical course of pallidal deep brain stimulation for phasic- and tonic-type cervical dystonia. Acta Neurochir 158:171–180
DeLong MR (1971) Activity of pallidal neurons during movement. J Neurophysiol 34:414–427
DeLong MR (1972) Activity of basal ganglia neurons during movement. Brain Res 40:127–135
DeLong MR, Wichmann T (2015) Basal ganglia circuits as targets for neuromodulation in Parkinson disease. JAMA Neurol 72:1354–1360
Eisinger RS, Cernera S, Gittis A, Gunduz A, Okun MS (2019) A review of basal ganglia circuits and physiology: application to deep brain stimulation. Parkinsonism Relat Disord
Goodman J, Packard MG (2018) The role of the dorsal striatum in extinction: a memory systems perspective. Neurobiol Learn Mem 150:48–55
Grips E, Blahak C, Capelle HH, Bazner H, Weigel R, Sedlaczek O, Krauss JK, Wohrle JC (2007) Patterns of reoccurrence of segmental dystonia after discontinuation of deep brain stimulation. J Neurol Neurosurg Psychiatry 78:318–320
Group ESoDiEC (2000) A prevalence study of primary dystonia in eight European countries. J Neurol 247:787–792
Huh R, Chung M (2016) Electrophysiological interpretations of the clinical response to stimulation parameters of pallidal deep brain stimulation for cervical dystonia. Acta Neurochir 158:2029–2038
Huh R, Song IU, Chung M (2018) Neuropsychological consequences of pallidal deep brain stimulation altering brain networks. J Clin Neurosci 54:50–56
Johnson MD, Miocinovic S, McIntyre CC, Vitek JL (2008) Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics 5:294–308
Krauss JK (2010) Surgical treatment of dystonia. Eur J Neurol 17(Suppl 1):97–101
Kroneberg D, Plettig P, Schneider GH, Kuhn AA (2018) Motor cortical plasticity relates to symptom severity and clinical benefit from deep brain stimulation in cervical dystonia. Neuromodulation 21:735–740
Li Q, Qian ZM, Arbuthnott GW, Ke Y, Yung WH (2014) Cortical effects of deep brain stimulation: implications for pathogenesis and treatment of Parkinson disease. JAMA Neurol 71:100–103
Liu X, Griffin IC, Parkin SG, Miall RC, Rowe JG, Gregory RP, Scott RB, Aziz TZ, Stein JF (2002) Involvement of the medial pallidum in focal myoclonic dystonia: a clinical and neurophysiological case study. Mov Disord 17:346–353
Liu X, Wang S, Yianni J, Nandi D, Bain PG, Gregory R, Stein JF, Aziz TZ (2008) The sensory and motor representation of synchronized oscillations in the globus pallidus in patients with primary dystonia. Brain 131:1562–1573
McCairn KW, Iriki A, Isoda M (2013) Deep brain stimulation reduces tic-related neural activity via temporal locking with stimulus pulses. J Neurosci 33:6581–6593
Meoni S, Fraix V, Castrioto A, Benabid AL, Seigneuret E, Vercueil L, Pollak P, Krack P, Chevrier E, Chabardes S, Moro E (2017) Pallidal deep brain stimulation for dystonia: a long term study. J Neurol Neurosurg Psychiatry 88:960–967
Meringolo M, Tassone A, Imbriani P, Ponterio G, Pisani A (2018) Dystonia: are animal models relevant in therapeutics? Rev Neurol (Paris) 174:608–614
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev 20:91–127
Quartarone A, Hallett M (2013) Emerging concepts in the physiological basis of dystonia. Mov Disord 28:958–967
Quartarone A, Pisani A (2011) Abnormal plasticity in dystonia: disruption of synaptic homeostasis. Neurobiol Dis 42:162–170
Ranck JB Jr (1975) Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98:417–440
Ruge D, Tisch S, Hariz MI, Zrinzo L, Bhatia KP, Quinn NP, Jahanshahi M, Limousin P, Rothwell JC (2011) Deep brain stimulation effects in dystonia: time course of electrophysiological changes in early treatment. Mov Disord 26:1913–1921
Udupa K, Chen R (2015) The mechanisms of action of deep brain stimulation and ideas for the future development. Prog Neurobiol 133:27–49
Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, Lagrange C, Tezenas du Montcel S, Dormont D, Grand S, Blond S, Detante O, Pillon B, Ardouin C, Agid Y, Destee A, Pollak P (2005) Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 352:459–467
Vinas-Guasch N, Wu YJ (2017) The role of the putamen in language: a meta-analytic connectivity modeling study. Brain Struct Funct 222:3991–4004
Volkmann J, Mueller J, Deuschl G, Kuhn AA, Krauss JK, Poewe W, Timmermann L, Falk D, Kupsch A, Kivi A, Schneider GH, Schnitzler A, Sudmeyer M, Voges J, Wolters A, Wittstock M, Muller JU, Hering S, Eisner W, Vesper J, Prokop T, Pinsker M, Schrader C, Kloss M, Kiening K, Boetzel K, Mehrkens J, Skogseid IM, Ramm-Pettersen J, Kemmler G, Bhatia KP, Vitek JL, Benecke R (2014) Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol 13:875–884
Walsh RA, Sidiropoulos C, Lozano AM, Hodaie M, Poon YY, Fallis M, Moro E (2013) Bilateral pallidal stimulation in cervical dystonia: blinded evidence of benefit beyond 5 years. Brain 136:761–769
Witt JL, Moro E, Ash RS, Hamani C, Starr PA, Lozano AM, Hodaie M, Poon YY, Markun LC, Ostrem JL (2013) Predictive factors of outcome in primary cervical dystonia following pallidal deep brain stimulation. Mov Disord 28:1451–1455
Yamada K, Hamasaki T, Hasegawa Y, Kuratsu J (2013) Long disease duration interferes with therapeutic effect of globus pallidus internus pallidal stimulation in primary cervical dystonia. Neuromodulation 16:219–225
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Functional Neurosurgery - Movement disorders
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Huh, R., Chung, M. Range of voluntary neck motility predicts outcome of pallidal DBS for cervical dystonia. Acta Neurochir 161, 2491–2498 (2019). https://doi.org/10.1007/s00701-019-04076-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-04076-z