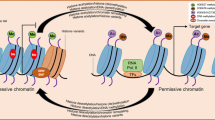
Abstract
Abiotic stresses are non-living factors with negative morphological and physiological effects on living organisms. Substantial evidence exists that gene expression changes during plant cell growth are regulated by chromatin reconfiguration and histone modification. Several types of histone modifications are dramatically transformed in stress-responsive gene regions under drought stress conditions. Environmental stresses also cause the root apical meristem (RAM) region to decelerate root growth. In this study, we investigated how quantitative changes in epigenetic markers in this region influence rice morphology and physiology. Both iron and salinity treatments changed the epigenetic landscape from euchromatic to heterochromatic according to heterochromatin (H3K9me2) and euchromatin (H3K4me) markers, especially in the proximal meristem region. Moreover, supplementation with external abscisic acid (ABA) was able to mimic the effect of environmental stresses on global epigenetic changes. In contrast, the addition of external auxin (IAA) to rice under saline conditions affected heterochromatin formation without influencing euchromatin transformation. Chromatin dynamics is therefore believed to be directly connected to plant growth regulator signaling. We discuss insights into the role of plant growth regulators: ABA and IAA, peroxide signaling, and their effects on the global epigenetic change of histone modification under abiotic stresses.





Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- IAA:
-
Indole-3-acetic acid
- RAM:
-
Root apical meristem
- H3K9me2:
-
Histone H3 dimethylation at lysine 9
- H3K4me2:
-
Histone H3 dimethylation at lysine 4
References
Atulba SL, Gutierrez J, Kim GW, Kim SY, Khan MI, Lee YB, Kim PJ (2015) Evaluation of rice root oxidizing potential using digital image analysis. J Korean Soc Appl Biol Chem 58:463–471
Berger SL (2002) Histone modifications in transcriptional regulation. Curr Opin Genet Dev 12:142–148
Braszewska-Zalewska A, Hasterok R (2013) Epigenetic modifications of nuclei differ between root meristematic tissues of Hordeum vulgare. Plant Signal Behav 8:2–4
Chen CC, Dixon JB, Turner FT (1980) Iron coatings on rice roots: morphology and models of development1. Soil Sci Soc Am J 44:1113–1119
Chen Z, Zang J, Whetstine J, Hong X, Davrazou F, Kutateladze TG, Zhang G (2006) Structural insights into histone demethylation by JMJD2 family members. Cell 125:691–702
Chinnusamy V, Zhu JK (2009) Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol 12:133–139
Chinnusamy V, Gong Z, Zhu J-K (2008) Abscisic acid-mediated epigenetic processes in plant development and stress responses. J Integr Plant Biol 50:1187–1195
Costas C, Desvoyes B, Gutierrez C (2011) A chromatin perspective of plant cell cycle progression. Biochim Biophys Acta - Gene Regul Mech 1809(8):379–387
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. In Annual Review of Plant Biology 61:651–679
Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Natl Acad Sci 108:18506–18511
Fischle W, Wang Y, Allis CD (2003) Histone and chromatin cross-talk. Curr Opin Cell Biol 15:172–183
Fuchs J, Demidov D, Houben A, Schubert I (2006) Chromosomal histone modification patterns—from conservation to diversity. Trends Plant Sci 11:199–208
Grafi G, Florentin A, Ransbotyn V, Morgenstern Y (2011) The stem cell state in plant development and in response to stress. Front Plant Sci 2:1–10
Gulnaz A, Iqbal J, Azam F (1999) Seed treatment with growth regulators and crop produktivity. II. Response of critical growth stages of wheat (Triticum aestivum L.) under salinity stress. Cereal Res 27:419–426
Jasencakova Z, Meister A, Schubert I (2001) Chromatin organization and its relation to replication and histone acetylation during the cell cycle in barley. Chromosoma 110:83–92
Jenuwein T, Allis CD (2001) Translating the histone code. Science 293:1074–1080
Ji H, Liu L, Li K, Xie Q, Wang Z, Zhao X, Li X (2014) PEG-mediated osmotic stress induces premature differentiation of the root apical meristem and outgrowth of lateral roots in wheat. J Exp Bot 65:4863–4872
Kim J-M, Sasaki T, Ueda M, Sako K, Seki M (2015) Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front Plant Sci 6:1–12
Lee SC, Luan S (2012) ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ 35:53–60
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2˙−, H2O2, and ˙OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Lobréaux S, Hardy T, Briat JF (1993) Abscisic acid is involved in the iron-induced synthesis of maize ferritin. EMBO J 12:651–657
Miller GAD, Suzuki N, Ciftci-yilmaz S, Mittler RON (2010) Reactive oxygen species homeostasis and signalling. Plant Cell Environ 33:453–467
Molina O, Vargiu G, Abad MA, Zhiteneva A, Jeyaprakash AA, Masumoto H, Earnshaw WC (2016) Epigenetic engineering reveals a balance between histone modifications and transcription in kinetochore maintenance. Nat Commun 7:13334
Pekowska A, Benoukraf T, Ferrier P, Spicuglia S (2010) A unique H3K4me2 profile marks tissue-specific gene regulation. Genome Res 20:1493–1502
Petruk S, Sedkov Y, Johnston DM, Hodgson JW, Black KL, Kovermann SK, Mazo A (2012) TrxG and PcG proteins but not methylated histones remain associated with DNA through replication. Cell 150:922–933
Rosa S, Ntoukakis V, Ohmido N, Pendle A, Abranches R, Shaw P (2014) Cell differentiation and development in Arabidopsis are associated with changes in histone dynamics at the single-cell level. Plant Cell 26:4821–4833
Sacks MM, Silk WK, Burman P (1997) Effect of water stress on cortical cell division rates within the apical meristem of primary roots of maize. Plant Physiol 114:519–527
Seto E, Yoshida M (2014) Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol 6:18713–18713
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Shi Y (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119:941–953
Strahl BD, Allis CD (2000) The language of covalent histone modifications. Nature 403:41–45
Turner BM (2002) Cellular memory and the histone code. Cell 111:285–291
Vaughn MW, Dedhia N, Mccombie WR, Lavine K, Mittal V, May B, Martienssen R (2004) Role of transposable elements in heterochromatin and epigenetic control. Nature 430:471–476
Verslues PE, Zhu J-K (2005) Before and beyond ABA: upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochem Soc Trans 33:375–379
Veselov D, Akhiyarova G, Kudoyarova G, Fricke W (2004) Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J Exp Bot 55:1115–1123
West G, Inzé D, Beemster GTS (2004) Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol 135:1050–1058
Yan S, Zhang Q, Li Y, Huang Y, Zhao L, Tan J, Li L (2014) Comparison of chromatin epigenetic modification patterns among root meristem, elongation and maturation zones in maize (Zea mays L.). Cytogenet Genome Res 143:179–188
Yang F, Zhang L, Li J, Huang J, Wen R, Ma L, Li L (2010) Trichostatin A and 5-azacytidine both cause an increase in global histone H4 acetylation and a decrease in global DNA and H3K9 methylation during mitosis in maize. BMC Plant Biol 10:178
Yang L, Zhang J, He J, Qin Y, Hua D, Duan Y, Gong Z (2014) ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLoS Genet 10(12):e1004791
Yoshida T, Mogami J, Yamaguchi-Shinozaki K (2014) ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr Opin Plant Biol 21:133–139
Zhang L, Hu Y, Yan S, Li H, He S, Huang M, Li L (2012) ABA-mediated inhibition of seed germination is associated with ribosomal DNA chromatin condensation, decreased transcription, and ribosomal RNA gene hypoacetylation. Plant Mol Biol 79:285–293
Zhao K, Tung C-W, Eizenga GC, Wright MH, Ali ML, Price AH, McCouch SR (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Commun 2:467
Zheng C, Halaly T, Acheampong AK, Takebayashi Y, Jikumaru Y, Kamiya Y, Or E (2015) Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J Exp Bot 66:1527–1542
Acknowledgments
This work was supported by the SmartD program (IAARD-Indonesian Ministry of Agriculture) to AP, and JSPS and CAS under the Japan-Czech Research Cooperative Program to NO. We thank Edanz Group (www.edanzediting.com/ac) for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
NO conceived of the study, participated in its design and coordination, and drafted the manuscript. AP carried out collected experiments, assembly of data, performed statistical analysis studies, and drafted the manuscript. WE prepared the rice materials and assisted with data interpretation. All authors read and NO approved the final manuscript.
Corresponding author
Additional information
Responsible Editor: Hans de Jong
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 11041 kb)
Rights and permissions
About this article
Cite this article
Polosoro, A., Enggarini, W. & Ohmido, N. Global epigenetic changes of histone modification under environmental stresses in rice root. Chromosome Res 27, 287–298 (2019). https://doi.org/10.1007/s10577-019-09611-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-019-09611-3