Abstract
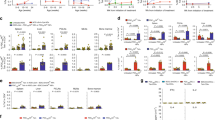
The considerable variability of responses amongst subjects to disease triggers and immunotherapies is a major obstacle to designing better immune-based therapies. Therefore, development of patient-tailored precision medicine that improves the efficacy of immunomodulatory drugs is necessary. The individualized response to disease triggers and immunomodulatory therapies was studied using the concanavalin A (ConA) immune-mediated hepatitis model and the oral administration of anti CD3 or β-glucosylceramide (GC). Mice were treated with anti-CD3 antibodies or GC followed by an injection of ConA. The effects of these treatments on liver damage and the immune profile were then analyzed. An individualized response to ConA and orally administered immunomodulatory agents was observed in eight consecutive experiments. While alleviation of the immune-mediated liver injury, as measured by serum levels of liver enzymes, was seen, and high intra-group and inter-experimental variabilities were detected. A similar individualized response was observed for the effect on serum levels of IFN-γ, TNF-α, and IL-10 and on CD4+CD25+, CD8+CD25+, and CD3+NK1.1+ lymphocytes. A personalized form of inherent randomness in an isolated system was documented, which may underlie the variability in responses to immune triggers and immunomodulatory therapies. The data support the use of personalized randomness-based platforms for improving the response to chronic therapies.





Similar content being viewed by others
Abbreviations
- GC:
-
β-glucosylceramide
- ConA:
-
Concanavalin A
- NKT:
-
Natural killer T
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- IFN-γ:
-
Interferon gamma
- TNF-α:
-
Tumor necrosis factor alpha
- IL10:
-
Interleukin 10
- PDL1:
-
Programmed death-ligand 1
- PD:
-
Programmed death-1
References
Kucuksezer UC, Ozdemir C, Akdis M, Akdis CA. Precision/Personalized Medicine in Allergic Diseases and Asthma. Arch Immunol Ther Exp. 2018;66:431–42. https://doi.org/10.1007/s00005-018-0526-6.
Doestzada M, Vila AV, Zhernakova A, Koonen DPY, Weersma RK, Touw DJ, et al. Pharmacomicrobiomics: a novel route towards personalized medicine? Protein Cell. 2018;9(5):432–45. https://doi.org/10.1007/s13238-018-0547-2.
Currie G, Delles C. Precision Medicine and Personalized Medicine in Cardiovascular Disease. Adv Exp Med Biol. 2018;1065:589–605. https://doi.org/10.1007/978-3-319-77932-4_36.
Tavakolpour S. Towards personalized medicine for patients with autoimmune diseases: Opportunities and challenges. Immunol Lett. 2017;190:130–8. https://doi.org/10.1016/j.imlet.2017.08.002.
Doria A, Gershwin ME, Selmi C. From old concerns to new advances and personalized medicine in lupus: The end of the tunnel is approaching. J Autoimmun. 2016;74:1–5. https://doi.org/10.1016/j.jaut.2016.08.007.
Biesen R, Rose T, Hoyer BF, Alexander T, Hiepe F. Autoantibodies, complement and type I interferon as biomarkers for personalized medicine in SLE. Lupus. 2016;25(8):823–9. https://doi.org/10.1177/0961203316640922.
Bauer C, Stec K, Glintschert A, Gruden K, Schichor C, Or-Guil M, et al. BioMiner: Paving the Way for Personalized Medicine. Cancer Informat. 2015;14:55–63. https://doi.org/10.4137/CIN.S20910.
Wardill HR, Tissing WJE. Determining risk of severe gastrointestinal toxicity based on pretreatment gut microbial community in patients receiving cancer treatment: a new predictive strategy in the quest for personalized cancer medicine. Curr Opin Support Palliat Care. 2017;11(2):125–32. https://doi.org/10.1097/SPC.0000000000000265.
Tiegs G. Cellular and cytokine-mediated mechanisms of inflammation and its modulation in immune-mediated liver injury. Z Gastroenterol. 2007;45(1):63–70.
Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45(2):475–85.
Margalit M, Ghazala SA, Alper R, Elinav E, Klein A, Doviner V, et al. Glucocerebroside treatment ameliorates ConA hepatitis by inhibition of NKT lymphocytes. Am J Physiol Gastrointest Liver Physiol. 2005;289(5):G917–25.
Ogura M, Deng S, Preston-Hurlburt P, Ogura H, Shailubhai K, Kuhn C, et al. Oral treatment with foralumab, a fully human anti-CD3 monoclonal antibody, prevents skin xenograft rejection in humanized mice. Clin Immunol. 2017;183:240–6. https://doi.org/10.1016/j.clim.2017.07.005.
Kuhn C, Weiner HL. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy. 2016;8(8):889–906. https://doi.org/10.2217/imt-2016-0049.
da Cunha AP, Weiner HL. Induction of immunological tolerance by oral anti-CD3. Clin Dev Immunol. 2012;2012:425021. https://doi.org/10.1155/2012/425021.
Wu HY, Maron R, Tukpah AM, Weiner HL. Mucosal anti-CD3 monoclonal antibody attenuates collagen-induced arthritis that is associated with induction of LAP+ regulatory T cells and is enhanced by administration of an emulsome-based Th2-skewing adjuvant. J Immunol. 2010;185(6):3401–7. https://doi.org/10.4049/jimmunol.1000836.
Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107(21):9765–70. https://doi.org/10.1073/pnas.0908771107.
Abraham M, Karni A, Dembinsky A, Miller A, Gandhi R, Anderson D, et al. In vitro induction of regulatory T cells by anti-CD3 antibody in humans. J Autoimmun. 2008;30(1-2):21–8. https://doi.org/10.1016/j.jaut.2007.11.007.
Ilan Y, Shailubhai K, Sanyal A. Immunotherapy with oral administration of humanized anti-CD3 monoclonal antibody: a novel gut-immune system-based therapy for metaflammation and NASH. Clin Exp Immunol. 2018;193(3):275–83. https://doi.org/10.1111/cei.13159.
Ilan Y. Review article: novel methods for the treatment of non-alcoholic steatohepatitis - targeting the gut immune system to decrease the systemic inflammatory response without immune suppression. Aliment Pharmacol Ther. 2016;44(11-12):1168–82. https://doi.org/10.1111/apt.13833.
Ilan Y, Zigmond E, Lalazar G, Dembinsky A, Ben Ya'acov A, Hemed N, et al. Oral administration of OKT3 monoclonal antibody to human subjects induces a dose-dependent immunologic effect in T cells and dendritic cells. J Clin Immunol. 2010;30(1):167–77. https://doi.org/10.1007/s10875-009-9323-7.
Lalazar G, Mizrahi M, Turgeman I, Adar T, Ben Ya'acov A, Shabat Y, et al. Oral Administration of OKT3 MAb to Patients with NASH, Promotes Regulatory T-cell Induction, and Alleviates Insulin Resistance: Results of a Phase IIa Blinded Placebo-Controlled Trial. J Clin Immunol. 2015;35(4):399–407. https://doi.org/10.1007/s10875-015-0160-6.
Halota W, Ferenci P, Kozielewicz D, Dybowska D, Lisovoder N, Samira S, et al. Oral anti-CD3 immunotherapy for HCV-nonresponders is safe, promotes regulatory T cells and decreases viral load and liver enzyme levels: results of a phase-2a placebo-controlled trial. J Viral Hepat. 2015;22(8):651–7. https://doi.org/10.1111/jvh.12369.
Ilan Y. Compounds of the sphingomyelin-ceramide-glycosphingolipid pathways as secondary messenger molecules: new targets for novel therapies for fatty liver disease and insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2016;310(11):G1102–17. https://doi.org/10.1152/ajpgi.00095.2016.
Zigmond E, Tayer-Shifman O, Lalazar G, Ben Ya'acov A, Weksler-Zangen S, Shasha D, et al. beta-glycosphingolipids ameliorated non-alcoholic steatohepatitis in the Psammomys obesus model. J Inflamm Res. 2014;7:151–8. https://doi.org/10.2147/JIR.S50508.
Zigmond E, Zangen SW, Pappo O, Sklair-Levy M, Lalazar G, Zolotaryova L, et al. Beta-glycosphingolipids improve glucose intolerance and hepatic steatosis of the Cohen diabetic rat. Am J Physiol. 2009;296(1):E72–8. https://doi.org/10.1152/ajpendo.90634.2008.
Zhang W, Moritoki Y, Tsuneyama K, Yang GX, Ilan Y, Lian ZX, et al. Beta-glucosylceramide ameliorates liver inflammation in murine autoimmune cholangitis. Clin Exp Immunol. 2009;157(3):359–64. https://doi.org/10.1111/j.1365-2249.2009.03971.x.
Shuvy M, Ben Ya'acov A, Zolotarov L, Lotan C, Ilan Y. Beta glycosphingolipids suppress rank expression and inhibit natural killer T cell and CD8+ accumulation in alleviating aortic valve calcification. Int J Immunopathol Pharmacol. 2009;22(4):911–8. https://doi.org/10.1177/039463200902200406.
Lalazar G, Ben Ya'acov A, Livovsky DM, El Haj M, Pappo O, Preston S, et al. Beta-glycoglycosphingolipid-induced alterations of the STAT signaling pathways are dependent on CD1d and the lipid raft protein flotillin-2. Am J Pathol. 2009;174(4):1390–9. https://doi.org/10.2353/ajpath.2009.080841.
Ben Ya'acov A, Lalazar G, Livovsky DM, Kanovich D, Axelrod E, Preston S, et al. Decreased STAT-1 phosphorylation by a thio analogue of beta-D-glucosylceramide is associated with altered NKT lymphocyte polarization. Mol Immunol. 2009;47(2-3):526–33. https://doi.org/10.1016/j.molimm.2009.07.030.
Zigmond E, Shalev Z, Pappo O, Alper R, Zolotarov L, Ilan Y. NKT lymphocyte polarization determined by microenvironment signaling: a role for CD8+ lymphocytes and beta-glycosphingolipids. J Autoimmun. 2008;31(2):188–95. https://doi.org/10.1016/j.jaut.2008.07.003.
Livovsky DM, Lalazar G, Ben Ya'acov A, Pappo O, Preston S, Zolotaryova L, et al. Administration of beta-glycolipids overcomes an unfavorable nutritional dependent host milieu: a role for a soy-free diet and natural ligands in intrahepatic CD8+ lymphocyte trapping and NKT cell redistribution. Int Immunopharmacol. 2008;8(9):1298–305. https://doi.org/10.1016/j.intimp.2008.05.005.
Lalazar G, Ben Ya'acov A, Eliakim-Raz N, Livovsky DM, Pappo O, Preston S, et al. Beta-glycosphingolipids-mediated lipid raft alteration is associated with redistribution of NKT cells and increased intrahepatic CD8+ T lymphocyte trapping. J Lipid Res. 2008;49(9):1884–93. https://doi.org/10.1194/jlr.M800113-JLR200.
Zigmond E, Preston S, Pappo O, Lalazar G, Margalit M, Shalev Z, et al. Beta-glucosylceramide: a novel method for enhancement of natural killer T lymphoycte plasticity in murine models of immune-mediated disorders. Gut. 2007;56(1):82–9.
Lalazar G, Zigmond E, Weksler-Zangen S, Ya'acov AB, Levy MS, Hemed N, et al. Oral Administration of beta-Glucosylceramide for the Treatment of Insulin Resistance and Nonalcoholic Steatohepatitis: Results of a Double-Blind. Placebo-Controlled Trial J Med Food. 2017;20(5):458–64. https://doi.org/10.1089/jmf.2016.3753.
Ilan Y, Ohana M, Pappo O, Margalit M, Lalazar G, Engelhardt D, et al. Alleviation of acute and chronic graft-versus-host disease in a murine model is associated with glucocerebroside-enhanced natural killer T lymphocyte plasticity. Transplantation. 2007;83(4):458–67.
Lalazar G, Preston S, Zigmond E, Ben Yaacov A, Ilan Y. Glycolipids as immune modulatory tools. Mini-Rev Med Chem. 2006;6(11):1249–53.
Safadi R, Zigmond E, Pappo O, Shalev Z, Ilan Y. Amelioration of hepatic fibrosis via beta-glucosylceramide-mediated immune modulation is associated with altered CD8 and NKT lymphocyte distribution. Int Immunol. 2007;19(8):1021–9.
Lalazar G, Ben Ya'acov A, Lador A, Livovsky DM, Pappo O, Preston S, et al. Modulation of intracellular machinery by beta-glycolipids is associated with alteration of NKT lipid rafts and amelioration of concanavalin-induced hepatitis. Mol Immunol. 2008;45(13):3517–25. https://doi.org/10.1016/j.molimm.2008.05.009.
Mizrahi M, Lalazar G, Ben Ya'acov A, Livovsky DM, Horowitz Y, Zolotarov L, et al. Beta-glycoglycosphingolipid-induced augmentation of the anti-HBV immune response is associated with altered CD8 and NKT lymphocyte distribution: a novel adjuvant for HBV vaccination. Vaccine. 2008;26(21):2589–95. https://doi.org/10.1016/j.vaccine.2008.03.026.
Elinav E, Pappo O, Sklair-Levy M, Margalit M, Shibolet O, Gomori M, et al. Amelioration of non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice by oral immune regulation towards liver-extracted proteins is associated with elevated intrahepatic NKT lymphocytes and serum IL-10 levels. J Pathol. 2006;208(1):74–81. https://doi.org/10.1002/path.1869.
Dennert G, Aswad F. The role of NKT cells in animal models of autoimmune hepatitis. Crit Rev Immunol. 2006;26(5):453–73.
Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117(8):2302–12.
Kawamura T, Takeda K, Kaneda H, Matsumoto H, Hayakawa Y, Raulet DH, et al. NKG2A inhibits invariant NKT cell activation in hepatic injury. J Immunol. 2009;182(1):250–8.
Hov JR, Karlsen TH. The Microbiome in Primary Sclerosing Cholangitis: Current Evidence and Potential Concepts. Semin Liver Dis. 2017;37(4):314–31. https://doi.org/10.1055/s-0037-1608801.
Goulart LR, Santos PS, Carneiro AP, Santana BB, Vallinoto AC, Araujo TG. Unraveling Antibody Display: Systems Biology and Personalized Medicine. Curr Pharm Des. 2016;22(43):6560–76. https://doi.org/10.2174/1381612822666160923112816.
Aabakken L, Karlsen TH, Albert J, Arvanitakis M, Chazouilleres O, Dumonceau JM, et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy. 2017;49(6):588–608. https://doi.org/10.1055/s-0043-107029.
Li S, Todor A, Luo R. Blood transcriptomics and metabolomics for personalized medicine. Comput Struct Biotechnol J. 2016;14:1–7. https://doi.org/10.1016/j.csbj.2015.10.005.
Sirotti S, Generali E, Ceribelli A, Isailovic N, De Santis M, Selmi C. Personalized medicine in rheumatology: the paradigm of serum autoantibodies. Auto Immun Highlights. 2017;8(1):10–3. https://doi.org/10.1007/s13317-017-0098-1.
Chen P, Huang NT, Chung MT, Cornell TT, Kurabayashi K. Label-free cytokine micro- and nano-biosensing towards personalized medicine of systemic inflammatory disorders. Adv Drug Deliv Rev. 2015;95:90–103. https://doi.org/10.1016/j.addr.2015.09.005.
Brzustewicz E, Bzoma I, Daca A, Szarecka M, Bykowska MS, Witkowski JM, et al. Heterogeneity of the cytokinome in undifferentiated arthritis progressing to rheumatoid arthritis and its change in the course of therapy. Move toward personalized medicine. Cytokine. 2017;97:1–13. https://doi.org/10.1016/j.cyto.2017.05.012.
Rodriguez-Carrio J, Lopez P, Suarez A. EPC dysfunction and immune networks: translating opportunities for the clinical setting in personalized medicine. Curr Med Chem. 2017. https://doi.org/10.2174/0929867324666170606101823.
Sui H, Ma N, Wang Y, Li H, Liu X, Su Y, et al. Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies. J Immunol Res. 2018;2018:6984948. https://doi.org/10.1155/2018/6984948.
Ilan Y. Advanced Tailored Randomness: A Novel Approach for Improving the Efficacy of Biological Systems. J Comput Biol. 2019. https://doi.org/10.1089/cmb.2019.0231.
Ilan Y. Why targeting the microbiome is not so successful: can randomness overcome the adaptation that occurs following gut manipulation? Clin Exp Gastroenterol. 2019;12:209–17. https://doi.org/10.2147/CEG.S203823.
Ilan Y. beta-Glycosphingolipids as Mediators of Both Inflammation and Immune Tolerance: A Manifestation of Randomness in Biological Systems. Front Immunol. 2019;10:1143. https://doi.org/10.3389/fimmu.2019.01143.
Ilan Y. Randomness in microtubule dynamics: an error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol Int. 2019;43(7):739–48. https://doi.org/10.1002/cbin.11157.
Ilan Y. Generating randomness: making the most out of disordering a false order into a real one. J Transl Med. 2019;17(1):49–12. https://doi.org/10.1186/s12967-019-1798-2.
Buiatti M, Buiatti M. Chance vs. necessity in living systems: a false antinomy. Riv Biol. 2008;101(1):29–66.
Montevil M, Mossio M, Pocheville A, Longo G. Theoretical principles for biology: Variation. Prog Biophys Mol Biol. 2016;122(1):36–50. https://doi.org/10.1016/j.pbiomolbio.2016.08.005.
Buiatti M, Longo G. Randomness and multilevel interactions in biology. Theory Biosci. 2013;132(3):139–58. https://doi.org/10.1007/s12064-013-0179-2.
Longo G, Montevil M, Sonnenschein C, Soto AM. In search of principles for a Theory of Organisms. J Biosci. 2015;40(5):955–68.
Longo G, Montevil M, Pocheville A. From bottom-up approaches to levels of organization and extended critical transitions. Front Physiol. 2012;3:232. https://doi.org/10.3389/fphys.2012.00232.
Vinks AA. Precision Medicine-Nobody Is Average. Clin Pharmacol Ther. 2017;101(3):304–7. https://doi.org/10.1002/cpt.600.
West J. Where Medicine Went Wrong: Rediscovering the Path to Complexity: Studies of Nonlinear Phenomena in Life Science. 2006.
Wu L, Jiang Z, Li C, Shu M. Prediction of heart rate variability on cardiac sudden death in heart failure patients: a systematic review. Int J Cardiol. 2014;174(3):857–60. https://doi.org/10.1016/j.ijcard.2014.04.176.
Sima CA, Inskip JA, Sheel AW, van Eeden SF, Reid WD, Camp PG. The reliability of short-term measurement of heart rate variability during spontaneous breathing in people with chronic obstructive pulmonary disease. Rev Port Pneumol (2006). 2017;23(6):338–42. https://doi.org/10.1016/j.rppnen.2017.06.001.
Dingwell JB, John J, Cusumano JP. Do humans optimally exploit redundancy to control step variability in walking? PLoS Comput Biol. 2010;6(7):e1000856. https://doi.org/10.1371/journal.pcbi.1000856.
Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114(10):1379–88.
Motulsky HJ. Common misconceptions about data analysis and statistics. Pharmacol Res Perspect. 2015;3(1):e00093. https://doi.org/10.1002/prp2.93.
Robertson D, Cook D. Unrealistic statistics: how average constitutive coefficients can produce non-physical results. J Mech Behav Biomed Mater. 2014;40:234–9. https://doi.org/10.1016/j.jmbbm.2014.09.006.
Denny M. The fallacy of the average: on the ubiquity, utility and continuing novelty of Jensen's inequality. J Exp Biol. 2017;220(Pt 2):139–46. https://doi.org/10.1242/jeb.140368.
Song YS, Patil A, Murphy EE, Slatkin M. Average probability that a "cold hit" in a DNA database search results in an erroneous attribution. J Forensic Sci. 2009;54(1):22–7. https://doi.org/10.1111/j.1556-4029.2008.00917.x.
Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The Fallacy of Average: How Using HbA1c Alone to Assess Glycemic Control Can Be Misleading. Diabetes Care. 2017;40(8):994–9. https://doi.org/10.2337/dc17-0636.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal experiments were carried out according to the guidelines of the Hebrew University-Hadassah Institutional Committee for the Care and Use of Laboratory Animals and with the committee’s approval.
Conflict of interest
YI is a founder of Oberon Sciences and consultant for Teva, ENZO, Protalix, Betalin Therapeutics, Immuron, SciM, Natural Shield, Tiziana Pharma, Plantylight, and Exalenz Bioscience.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLS 85 kb)
Rights and permissions
About this article
Cite this article
El-Haj, M., Kanovitch, D. & Ilan, Y. Personalized inherent randomness of the immune system is manifested by an individualized response to immune triggers and immunomodulatory therapies: a novel platform for designing personalized immunotherapies. Immunol Res 67, 337–347 (2019). https://doi.org/10.1007/s12026-019-09101-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-019-09101-y