Abstract
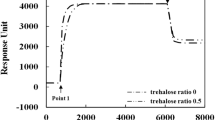
The behavior of the cinnamycin immobilized on the gold nanorod(AuNR) was investigated using surface plasmon resonance(SPR). For the comparison of the immobilized cinnamycin, the study for the free cinnamycin was also conducted. The bilayer was fabricated by tethering 1,2-dipalmitoyl-sn-glycero-3-phosphothioethanols on a gold surface to form a monolayer and then using liposomes to adsorb an outer layer on the tethered-monolayer. The liposomes were prepared with a desired ratio of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine to 1,2-dioleoyl-sn-glycero-3-phosphocholine (0:100, 5:95, 10:90, 20:80, and 30:70). After the cinnamycin was injected on the bilayers, the specific binding between the cinnamycin and the bilayer was monitored with SPR. The inclusion of DOPE in the outer layer clearly led to the specific binding of the cinnamycin on the membranes. Specifically, the binding behavior of the immobilized was different from that of the free. For the free cinnamycin, the binding amount of cinnamycin at 10% was two times more than that at 5%. For the immobilized cinnamycin, the amounts were identical for both compositions. However, the rate was much faster for the immobilized cinnamycin at 10% than 5%, compared to that for the free at both compositions. This difference was attributed to the mean-molecular areas of the cinnamycin and DOPE, and the steric effect of the AuNR. For the effects of the heat and storage, the immobilized enzyme showed less decrease in the relative binding amount than the free one.





Similar content being viewed by others
References
Chen PC, Mwakwari SC, Oyelere AK (2008) Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnol Sci Appl 1:45–65
Choung SY, Kobayashi T, Takemoto K, Ishitsuka H, Inoue K (1988) Interaction of a cyclic peptide, Ro09-0198, with phosphatidylethanolamine in liposomal membranes. Biochim Biophys Acta 940:180–187
Emoto K, Kobayashi T, Yamaji A, Aizawa H, Yahara I, Inoue K, Umeda M (1996) Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc Natl Acad Sci USA 93:12867–12872
Gennis RB (1989) Biomembranes: molecular structure and function. Springer, New York
Hosoda K, Ohya M, Kohno T, Tadakazu M, Shigeru E, Wakamatsu K (1996) Structure determination of an immunopotentiator peptide, cinnamycin, complexed with lysophosphatidylethanolamine by 1H-NMR. J Biochem 119:226–230
Lee S-R, Park J-W (2018) Trehalose-induced variation in physical properties of fluidic lipid bilayer. J Membr Biol 251:705–709
Liu K, Zheng Y, Lu X, Thai T, Lee NA, Bach U, Gooding JJ (2015) Biocompatible gold nanorods: one-step surface functionalization, highly colloidal stability, and low cytotoxicity. Langmuir 31:4973–4980
Machaidze G, Ziegler A, Seelig J (2002) Specific binding of Ro 09-0198 (cinnamycin) to phosphatidylethanolamine: a thermodynamic analysis. Biochemistry 41:1965–1971
Makino A, Baba T, Fujimoto K, Iwamoto K, Yano Y, Terada N, Ohno S, Sato SB, Ohta A, Umeda M, Matsuzaki K, Kobayashi T (2003) Cinnamycin (Ro 09-0198) promotes cell binding and toxicity by inducing transbilayer lipid movement. J Biol Chem 278:3204–3209
Meuse CW, Krueger S, Majkrzak CF, Dura JA, Fu J, Connor JT, Plant AL (1998) Hybrid bilayer membranes in air and water: infrared spectroscopy and neutron reflectivity studies. Biophys J 74:1388–1398
Ohlsson PÅ, Tjärnhage T, Herbai E, Löfås S, Puu G (1995) Liposome and proteoliposome fusion onto solid substrates, studied using atomic force microscopy, quartz crystal microbalance and surface plasmon resonance. Biological activities of incorporated components. Bioelectrochem Bioenerg 38:137–148
Park JW, Ahn DJ (2008) Temperature effect on nanometer-scale physical properties of mixed phospholipid monolayers. Colloids Surf B 62:157–161
Schmidt EK, Liebermann T, Kreiter M, Jonczyk A, Naumann R, Offenhausser A, Neumann E, Kukol A, Maelicke A, Knoll W (1998) Incorporation of the acetylcholine receptor dimer from Torpedo californica in a peptide supported lipid membrane investigated by surface plasmon and fluorescence spectroscopy. Biosens Bioelectron 13:585–591
Spector AA, Yorek MA (1985) Membrane lipid composition and cellular function. J Lipid Res 26:1015–1035
Stenberg E, Persson B, Roos H, Urbaniczky CJ (1991) Quantitative determination of surface concentration of protein with surface plasmon resonance using radiolabeled proteins. J Colloid Interface Sci 143:513–526
Terrettaz S, Stora T, Duschl C, Vogel H (1993) Protein binding to supported lipid membranes: investigation of the cholera toxin-ganglioside interaction by simultaneous impedance spectroscopy and surface plasmon resonance. Langmuir 9:1361–1369
Wakamatsu K, Choung SY, Kobayashi T, Inoue K, Higashijima T, Miyazawa T (1990) Complex formation of peptide antibiotic Ro09-0198 with lysophosphatidylethanolamine: 1H NMR analyses in dimethyl sulfoxide solution. Biochemistry 29:113–118
Zhang H, Chen G, Yu B, Cong H (2018) Emerging advanced nanomaterials for cancer photothermal therapy. Rev Adv Mater Sci 53:131–146
Acknowledgements
This study was supported by the Research Program funded by the SeoulTech (Seoul National University of Science and Technology). We thank all of the members of Department of Chemical and Biomolecular Engineering at the Seoul National University of Science and Technology for help and valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, G.S., Park, JW. Interactions of Cinnamycin-Immobilized Gold Nanorods with Biomimetic Membranes. J Membrane Biol 253, 37–42 (2020). https://doi.org/10.1007/s00232-019-00103-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-019-00103-3