Abstract
Purpose
To assess the efficacy and safety of a newly developed fully covered irradiation stent (FCIS) compared with a partially covered irradiation stent (PCIS) in patients with unresectable malignant dysphagia.
Materials and Methods
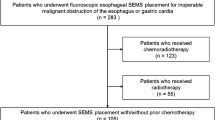
Data of 195 patients [158 (81.0%) males, median age of 75 years (range 49–89 years)] who underwent FCIS or PCIS placement for unresectable malignant dysphagia from January 2012 to November 2017 were retrospectively analyzed. The median follow-up time was 181 days (range 4–547 days). Outcomes were measured in terms of recurrent dysphagia (primary), technical success, clinical success, overall survival, and adverse events. Recurrent dysphagia was analyzed by Fine–Gray regression model.
Results
The technical success rate was 97.8% (87/89) in the FCIS group and 99.1% (105/106) in the PCIS group (P = 0.59). The clinical success rate was 100.0% in both groups. There was no statistically significant difference in the recurrent dysphagia rate between the FCIS and PCIS groups (21.8% vs. 28.6%; P = 0.12). Compared with PCISs, FCISs were associated with a decrease in tissue/tumor growth rate (11.5% vs. 21.9%; P = 0.01), while stent migration rates were statistically comparable (11.5% vs. 5.7%; P = 0.23). The median overall survivals were comparable between the FCIS and PCIS groups (164 days vs. 162 days; P = 0.70). A dysphagia score of 4 and metastasis were risk factors for survival. No significant differences were observed in the rates of adverse events, including chest pain, fistula formation, hemorrhage, and aspiration pneumonia (P > 0.05).
Conclusion
For patients with unresectable malignant dysphagia, this newly developed FCIS can provide efficacy and safety comparable to those of a PCIS. Compared with PCIS, this FCIS is more successful in preventing tissue/tumor growth, with a comparable stent migration rate.






Similar content being viewed by others
References
Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383–96. https://doi.org/10.1016/S0140-6736(17)31462-9.
Spaander MC, Baron TH, Siersema PD, Fuccio L, Schumacher B, Escorsell A, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2016;48(10):939–48. https://doi.org/10.1055/s-0042-114210.
Homs MY, Steyerberg EW, Eijkenboom WM, Tilanus HW, Stalpers LJ, Bartelsman JF, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet. 2004;364(9444):1497–504. https://doi.org/10.1016/S0140-6736(04)17272-3.
Zhongmin W, Xunbo H, Jun C, Gang H, Kemin C, Yu L, et al. Intraluminal radioactive stent compared with covered stent alone for the treatment of malignant esophageal stricture. Cardiovasc Interv Radiol. 2012;35(2):351–8. https://doi.org/10.1007/s00270-011-0146-6.
Guo JH, Teng GJ, Zhu GY, He SC, Fang W, Deng G, et al. Self-expandable esophageal stent loaded with 125I seeds: initial experience in patients with advanced esophageal cancer. Radiology. 2008;247(2):574–81. https://doi.org/10.1148/radiol.2472070999.
Zhu HD, Guo JH, Mao AW, Lv WF, Ji JS, Wang WH, et al. Conventional stents versus stents loaded with (125)iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. Lancet Oncol. 2014;15(6):612–9. https://doi.org/10.1016/S1470-2045(14)70131-7.
Didden P, Reijm AN, Erler NS, Wolters LMM, Tang TJ, Ter Borg PCJ, et al. Fully vs. partially covered self-expandable metal stent for palliation of malignant esophageal strictures: a randomized trial (the COPAC study). Endoscopy. 2018;50(10):961–71. https://doi.org/10.1055/a-0620-8135.
van Heel NC, Haringsma J, Boot H, Cats A, Vanhoutvin SA, Kuipers EJ. Comparison of 2 expandable stents for malignant esophageal disease: a randomized controlled trial. Gastrointest Endosc. 2012;76(1):52–8. https://doi.org/10.1016/j.gie.2012.02.050.
van Boeckel PG, Repici A, Vleggaar FP, Solito B, Rando G, Cortelezzi C, et al. A new metal stent with a controlled-release system for palliation of malignant dysphagia: a prospective, multicenter study. Gastrointest Endosc. 2010;71(3):455–60. https://doi.org/10.1016/j.gie.2009.09.046.
Hourneaux de Moura EG, Toma K, Goh KL, Romero R, Dua KS, Felix VN, et al. Stents for benign and malignant esophageal strictures. Ann N Y Acad Sci. 2013;1300:119–43. https://doi.org/10.1111/nyas.12242.
Reijm AN, Didden P, Schelling SJC, Siersema PD, Bruno MJ, Spaander MCW. Self-expandable metal stent placement for malignant esophageal strictures—changes in clinical outcomes over time. Endoscopy. 2019;51(1):18–29. https://doi.org/10.1055/a-0644-2495.
Seven G, Irani S, Ross AS, Gan SI, Gluck M, Low D, et al. Partially versus fully covered self-expanding metal stents for benign and malignant esophageal conditions: a single center experience. Surg Endosc. 2013;27(6):2185–92. https://doi.org/10.1007/s00464-012-2738-x.
Ogilvie AL, Dronfield MW, Ferguson R, Atkinson M. Palliative intubation of oesophagogastric neoplasms at fibreoptic endoscopy. Gut. 1982;23(12):1060–7.
Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13(2 Pt 1):559–65. https://doi.org/10.1158/1078-0432.CCR-06-1210.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. https://doi.org/10.2307/2670170.
Hirdes MM, Vleggaar FP, de Beule M, Siersema PD. In vitro evaluation of the radial and axial force of self-expanding esophageal stents. Endoscopy. 2013;45(12):997–1005. https://doi.org/10.1055/s-0033-1344985.
Shim CS, Jung IS, Bhandari S, Ryu CB, Hong SJ, Kim JO, et al. Management of malignant strictures of the cervical esophagus with a newly-designed self-expanding metal stent. Endoscopy. 2004;36(6):554–7. https://doi.org/10.1055/s-2004-814555.
Repici A, Jovani M, Hassan C, Solito B, Di Mitri R, Buffoli F, et al. Management of inoperable malignant oesophageal strictures with fully covered WallFlex((R)) stent: a multicentre prospective study. Dig Liver Dis. 2014;46(12):1093–8. https://doi.org/10.1016/j.dld.2014.08.037.
Kim ES, Jeon SW, Park SY, Cho CM, Tak WY, Kweon YO, et al. Comparison of double-layered and covered Niti-S stents for palliation of malignant dysphagia. J Gastroenterol Hepatol. 2009;24(1):114–9. https://doi.org/10.1111/j.1440-1746.2008.05674.x.
Persson J, Smedh U, Johnsson A, Ohlin B, Sundbom M, Nilsson M, et al. Fully covered stents are similar to semi-covered stents with regard to migration in palliative treatment of malignant strictures of the esophagus and gastric cardia: results of a randomized controlled trial. Surg Endosc. 2017;31(10):4025–33. https://doi.org/10.1007/s00464-017-5441-0.
Fuccio L, Scagliarini M, Frazzoni L, Battaglia G. Development of a prediction model of adverse events after stent placement for esophageal cancer. Gastrointest Endosc. 2016;83(4):746–52. https://doi.org/10.1016/j.gie.2015.08.047.
Siddiqui AA, Sarkar A, Beltz S, Lewis J, Loren D, Kowalski T, et al. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc. 2012;76(1):44–51. https://doi.org/10.1016/j.gie.2012.02.036.
Mezes P, Krokidis ME, Katsanos K, Spiliopoulos S, Sabharwal T, Adam A. Palliation of esophageal cancer with a double-layered covered Nitinol stent: long-term outcomes and predictors of stent migration and patient survival. Cardiovasc Interv Radiol. 2014;37(6):1444–9. https://doi.org/10.1007/s00270-013-0829-2.
Chen HL, Shen WQ, Liu K. Radioactive self-expanding stents for palliative management of unresectable esophageal cancer: a systematic review and meta-analysis. Dis Esophagus. 2017;30(5):1–16. https://doi.org/10.1093/dote/dow010.
So H, Ahn JY, Han S, Jung K, Na HK, Lee JH, et al. Efficacy and safety of fully covered self-expanding metal stents for malignant esophageal obstruction. Dig Dis Sci. 2018;63(1):234–41. https://doi.org/10.1007/s10620-017-4839-9.
Acknowledgements
This study was funded by the Jiangsu Provincial Special Program of Social Development (BE2016783).
Funding
This study was funded by the Jiangsu Provincial Special Program of Social Development (BE2016783).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for Publication
For this type of study, consent for publication is not required.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This work was performed with the approval of the local ethics committee. For this type of study, formal consent is not required.
Informed Consent
Informed consent was obtained from all individual participants included in the study. This study was approved by our institutional review board, and the requirement for written informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, C., Lu, J., Wang, Y. et al. A New Fully Covered Irradiation Stent Versus a Partially Covered Irradiation Stent for Unresectable Malignant Dysphagia: A Single-Center Experience. Cardiovasc Intervent Radiol 42, 1142–1152 (2019). https://doi.org/10.1007/s00270-019-02252-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02252-3