Abstract
Background
Infliximab can prevent colectomy in patients hospitalized with acute severe ulcerative colitis (ASUC). In cases of ASUC, fecal losses of infliximab may lead to low drug levels and reduced efficacy.
Aim
To determine 90-day colectomy risk and postoperative complications in patients receiving single-dose and accelerated induction of infliximab for ASUC.
Methods
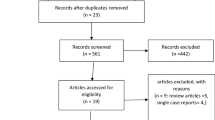
We conducted a retrospective review of patients hospitalized with ASUC requiring infliximab therapy between 2013 and 2017 at the University of Michigan. Patients were excluded if they had an enteric infection, received an anti-TNF previously, or received cyclosporine during the same admission. The primary outcome was colectomy within 90 days of admission. Patients receiving single-dose induction infliximab were compared to those receiving accelerated rescue induction with two doses of infliximab prior to day 14. Administration of accelerated induction was guided by a protocol, suggesting administering a second dose of infliximab to those with only a partial response in CRP 3 days after the initial dose. Postoperative outcomes including 30-day readmission rates and complications were compared using descriptive statistics.
Results
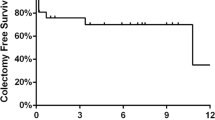
From 2013 to 2017, 66 patients with ASUC met our criteria. Thirty-three received accelerated induction (50.0%). The colectomy rate in the accelerated induction group was 30.3% versus 24.2% in the single-dose induction group (p = 0.58). There was no detected difference in postoperative outcomes between the accelerated and single-dose rescue induction.
Conclusions
In this retrospective review, 69.7% of those failing to respond to single-dose infliximab were able to avoid colectomy with an accelerated rescue induction strategy without worsening postoperative outcomes. Larger studies of accelerated dosing infliximab are needed.


Similar content being viewed by others
References
Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohn’s Colitis. 2010;4:431–437.
Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5:103–110.
Järnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805–1811.
Brandse JF, Van Den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology. 2015;1:350–355.e2.
Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2014;. https://doi.org/10.1016/j.cgh.2014.07.041.
Higgins PDR, Waljee AK, Kinnucan J, Aldrich L, Del Valle J, Nostrant TT, et al. University of michigan severe ulcerative colitis protocol [Internet]. 2017 Oct 10 [cited 2018 Apr 16]. http://www.med.umich.edu/ibd/docs/severeucprotocol.pdf.
Shah SC, Naymagon S, Panchal HJ, Sands BE, Cohen BL, Dubinsky MC. Accelerated infliximab dosing increases 30-day colectomy in hospitalized ulcerative colitis patients: a propensity score analysis. Inflamm Bowel Dis. 2018;15:651–659.
Choy MC, Seah D, Gorelik A, et al. Comparison of accelerated infliximab induction vs standard induction treatment in acute severe ulcerative colitis. Gastroenterology. 2016;12:S803.
Nalagatla N, Falloon K, Tran G, et al. Effect of accelerated infliximab induction on short- and long-term outcomes of acute severe ulcerative colitis: a retrospective multi-center study and meta-analysis. Clin Gastroenterol Hepatol. 2018;23:1–29.
Lee RH, Efron DT, Tantry U, et al. Inhibition of tumor necrosis factor-alpha attenuates wound breaking strength in rats. Wound Repair Regen. 2000;8:547–553.
Yang Z, Wu Q, Wu K, Fan D. Meta-analysis: pre-operative infliximab treatment and short-term post-operative complications in patients with ulcerative colitis. Aliment Pharmacol Ther. 2010;15:486–492.
Narula N, Charleton D, Marshall JK. Meta-analysis: peri-operative anti-TNFα treatment and post-operative complications in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:1057–1064.
Leeds IL, Truta B, Parian AM, et al. Early surgical intervention for acute ulcerative colitis is associated with improved postoperative outcomes. J Gastrointest Surg. 2017;21:1675–1682.
Feuerstein JD, Jiang ZG, Belkin E, et al. Surgery for ulcerative colitis is associated with a high rate of readmissions at 30 days. Inflamm Bowel Dis. 2015;21:2130–2136.
Funding
This work was supported by American Surgical Association Foundation Fellowship (5P50CA130810 and 5P30A046592 to KMH); John S. and Suzanne C. Munn Cancer Research Fund (KMH); and National Cancer Institute (K08CA190645 to KMH).
Author information
Authors and Affiliations
Contributions
Shail M. Govani was involved in conception, data collection/analysis, drafting article, and final approval. Jeffrey A. Berinstein was involved in data collection/analysis, drafting article, critical review of the manuscript, and final approval. Akbar K. Waljee was involved in conception, data analysis, critical review of the manuscript, and final approval. Ryan Stidham was involved in conception, critical review of the manuscript, and final approval. Peter D.R. Higgins was involved in conception, critical review of the manuscript, and final approval. Karin M. Hardiman was involved in conception, critical review of the manuscript, and final approval.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Ryan Stidham serves as a consultant to AbbVie. Dr. Peter Higgins serves as a consultant to AbbVie and Lycera. The authors have no other financial disclosures related to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Govani, S.M., Berinstein, J.A., Waljee, A.K. et al. Use of Accelerated Induction Strategy of Infliximab for Ulcerative Colitis in Hospitalized Patients at a Tertiary Care Center. Dig Dis Sci 65, 1800–1805 (2020). https://doi.org/10.1007/s10620-019-05957-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05957-0