Abstract
Social interaction involves self-initiated actions that engage subjective awareness of one's own volition. Individuals with social communication needs or social anxiety find it particularly difficult to initiate social interactions. However, extant studies have not specifically addressed how perceived exclusion may influence self-initiated actions during social interaction. As a first step to address this question, we scanned 24 healthy adults participating in a Cyberball game with two fictive players. By contrasting events of observing, receiving, and initiating ball toss during a scenario of fair game (FG) and of exclusion (EX), we examined the neural correlates of self-initiated action during social interactions. Behaviorally, participants were faster in catching but slower in tossing the ball in EX compared with FG, suggesting a burden during self-initiated actions during social exclusion. Tossing versus receiving (or observing) engaged higher activity during EX than FG in the precuneus and angular gyrus, regions that have been widely implicated in theory of mind processing and social emotions. Across subjects these cortical activities correlated positively with the difference between EX and FG in the percentage of trials where participants tossed the ball back to the same player (r = 0.69, p < 0.001). Together, the results suggested that, in healthy adults, social exclusion encumbered and engaged higher posterior cortical activations during self-initiated actions. The findings may facilitate future research of neural markers of social behavioral disorders.
Similar content being viewed by others
Introduction
In research of affective and social neuroscience, abundant work has examined cerebral responses to facial emotions (Fusar-Poli et al., 2009; Atkinson & Adolphs, 2011; Sabatinelli et al., 2011; Srinivasan, Golomb, & Martinez, 2016). Studies also have explored the neural processes of specific social interactions or emotions, such as interpretation of ambiguous social cues (Davis, Neta, Kim, Moran, & Whalen, 2016), sense of fairness and justice (Klapwijk et al., 2016), guilt and shame (Whittle, Liu, Bastin, Harrison, & Davey, 2016), hostility (Nakagawa et al., 2017), and retaliation (Emmerling et al., 2016), and how cerebral responses to social interaction vary with individual traits in approach and inhibition (Radke et al., 2016). Other work elucidated the neural processes of depression in relation to social threat processing (Jankowski et al., 2018) and the effects of mindfulness on the management of social rejection (Martelli, Chester, Warren Brown, Eisenberger, & Nathan DeWall, 2018). These findings provide important clues to the biological underpinnings of various dimensions of social interaction.
Many studies employed a Cyberball task (Eisenberger, Lieberman, & Williams, 2003; Williams & Jarvis, 2006) to investigate the neural correlates of social inclusion and exclusion in health (Gonzalez, Beckes, Chango, Allen, & Coan, 2014; van der Meulen et al., 2017; Wasylyshyn et al., 2018) and illness (Domsalla et al., 2013; Kumar et al., 2017). In the Cyberball tasks, by observing players tossing a ball to fellow players or with themselves engaged in the game, participants were involved in the dynamics of social interactions. Post-game debriefing suggested that exclusion in the Cyberball task induced significant feelings of ostracism (Hartgerink, van Beest, Wicherts, & Williams, 2015) and may be followed by retaliation of the participants by making lower offers to excluders in a Dictator game (Moor et al., 2012). Social exclusion engaged the insula, dorsal anterior cingulate cortex, and other prefrontal cortical regions, as well as the posterior cingulate cortex (Eisenberger et al., 2003; Kross, Egner, Ochsner, Hirsch, & Downey, 2007; Lieberman & Eisenberger, 2009; DeWall et al., 2010; Bolling et al., 2011; Sebastian et al., 2011; Moor et al., 2012; see Rotge et al., 2014 and Wang, Braun, & Enck, 2017 for a review). In contrast, fewer studies focused on social inclusion and the reported neural responses appeared less consistent (Brown et al., 2017; van der Meulen et al., 2017).
A critical component of social interaction involves self-initiated actions, which engage awareness of one's own volition. This experience of self-agency is critical to social interaction, because our actions are intended to influence other people, and a sense of self-agency is required to understand these influences. That is, the ability to “own” one’s action and monitor the outcomes of actions is central to social interaction (Koban & Pourtois, 2014). Self-agency enables understanding of others’ emotions during social interactions (Ruys & Aarts, 2012). Imagining self- versus third-person agency of actions that either conformed to or countered a social value elicited emotions with distinct cerebral responses (Zahn et al., 2008). An earlier study suggested that actions within a social compared with individualized context increased the auditory sensory attenuation effects as supported by self-agency (Weiss, Herwig, & Schütz-Bosbach, 2011). Together, these studies support a role of self-agency in social interactions and the influences of a social context on sensor-motor processes contingent on self-agency.
Initiation of social interaction can be challenging, particularly for those with complex communication needs (Carter, Davis, Klin, & Volkmar, 2005; Bishop, Gahagan, & Lord, 2007). Social skill trainings have aimed to improve the extent to which individuals with Autism Spectrum Disorders initiate peer interactions (Bauminger, 2002). Although social exclusion motivates interpersonal reconnection in neurotypical populations, those high in fear of negative evaluation may not respond to new interactions in an affiliative fashion (Maner, DeWall, Baumeister, & Schaller, 2007). Indeed, individuals with social anxiety often find it difficult to initiate social interactions. Imaging studies suggested that initiating joint attention, or directing another person's attention to an object, engaged cerebral processes that are distinct from those responding to joint attention (Redcay, Kleiner, & Saxe, 2012). Furthermore, an extensive body of research suggested that inferior parietal cortical, including angular gyrus activation reflected the experiences of agency by processing action signals generated in the frontal cortex (Renes, van Haren, Aarts, & Vink, 2015; Maurer et al., 2016; Voss, Chambon, Wenke, Kuhn, & Haggard, 2017). Other studies implicated the default mode network (Fukushima, Goto, Maeda, Kato, & Umeda, 2013; de Bezenac, Sluming, Gouws, & Corcoran, 2016; Spaniel et al., 2016). A meta-analysis suggested the role of the insula in self-agency across multiple behavioral tasks (Sperduti, Delaveau, Fossati, & Nadel, 2011). Using a paradigm that simulated the loss of self-agency in virtual reality and titrating the levels of mismatch, another study identified a wide swath of brain regions to support self-agency, including the posterior parietal cortex, superior temporal sulcus, precuneus, insula, and cerebellum (Nahab et al., 2011). Altogether, these studies suggest the potential importance in investigating the cerebral processes of self-initiated actions during social interaction.
On the other hand, little work to our knowledge has investigated the neural bases of self-initiated actions during social interaction. Extant imaging studies of the Cyberball task largely employed a block design and reported regional activations across inclusion and exclusion blocks without distinguishing activities during events that specifically engaged the participants. Thus, it remains unclear how one responds to tossing versus receiving a ball or observation and how these neural responses may be altered during social exclusion.
We addressed this gap of research. Twenty-four healthy adults participated in fMRI under three different conditions of a Cyberball task: observation (OB) where participants observed two fictive fellows playing; fair game (FG), where participants were equally involved in three-way interaction; and exclusion (EX), where participants were largely excluded from the game. We employed a mixed block-event design and identified brain activations specific to self-initiated actions. We hypothesized that distinct regional activations support ball toss and catch and that EX, compared with FG, will burden participants and involve greater activations during self-initiated actions in regions implicated in self-agency and theory-of-mind processing.
Materials and methods
Subjects and assessments
Twenty-four adult healthy volunteers (mean ± SD: 49 ± 16 years; 12 women) participated in the study. Participants underwent clinical screening and received urine toxicology tests at intake assessment and before imaging, as part of our study routine. All were required to be physically healthy with no major medical illnesses, current use of prescription medications, history of head injury or neurological illness, current or history of axis I disorders, as defined by the Diagnostic and Statistical Manual of Mental Disorders-IV, and showed negative urine test results. All participants were right-handed and used the right hand to respond in the behavioral task. Before the study, all participants signed an informed consent according to a protocol approved by the Human Investigation Committee at Yale University.
We used the Social Interaction Anxiety Scale (SIAS) to quantify social anxiety (Mattick & Clarke, 1998). The SIAS evaluates fears of general social interaction, shows excellent internal consistency and test-retest reliability, and captures treatment-related changes in anxiety. Each of the 19 items is scored from 0 to 4, with two items to be reversed scored. Factor analysis revealed one distinct factor—fear of social interaction—with all items showing high loading on the factor. All 19 items of the SIAS significantly distinguished social phobia from agoraphobia, simple phobia, and community samples. Individuals with social anxiety disorder showed a score of 33.4 ± 16.4 (mean ± SD) in women and 36.0 ± 16.5 in men compared with 19.4 ± 11.9 for women and 18.2 ± 11.7 for men in the community sample (Mattick & Clarke, 1998). The current cohort showed an SIAS score of 12.7 ± 7.5 and reflected a neurotypical, nonclinical population.
Experimental design and statistical analysis
Cyberball task
Participants were engaged in a variant of the Cyberball task (Williams & Jarvis, 2006) during fMRI (Fig. 1a). Participants were instructed to play a ball game via the Internet with two other individuals as named on the screen, who were fictive figures controlled by a computer program. Ball toss, catch, and drop were each accompanied by a distinct audio. Participants were instructed to do their best to catch the ball by estimating its arrival time and pressing a button; too early or a late response would result in a “drop” (see below). In turn, participants pressed one of two buttons to decide which of the two fictive figures to toss the ball to.
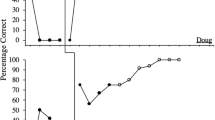
(a) Cyberball task. OB: observation; FG: fair game, with equal shares of catch and toss; EX: exclusion with ~1/12 ball toss to and from the participant and 0% catch. The numbers indicate the approximate % of ball toss among players. Cartoons were animated with sound effects in the task. Behavioral data in the Cyberball task include (b) reaction time (RT) in receiving the ball, (c) RT in tossing the ball, and (d) % of ball toss to the same player from which the participants just received the ball (i.e., same/total). Note that participants all attempted to catch the ball although the outcome (miss) was dictated by the program in EX sessions. Group mean ± S.E. as well as individual data are shown each for FG and EX. Participants were faster in catching the ball and slower in tossing the ball during EX than during FG. ***p < 0.001, two-tailed paired t test
There were three different scenarios: (1) observation (OB), in which participants were instructed to simply watch; (2) fair game (FG), in which participants received/tossed the ball approximately 1/3 of the time; and social exclusion (EX), in which participants were tossed the ball approximately 1/12 of the time. Thus, unlike the majority of the Cyberball studies, the current paradigm included some interaction trials in the EX scenario, so we could examine the neural processes involved in self-initiated actions. Individual sessions lasted 8 m each, separated by a break in between, with two sessions per scenario and following a fixed order: OB-break (~1 m)-FG-break-EX-break-OB-break-FG-break-EX. Within each session, a trial started with a ball toss initiated by a fictive player. The fictive player was animated to appear to think and look alternatingly at the participant and the other fictive player before tossing (2.0 to 16.0 s, uniform distribution). The ball traveled at different speeds for 3.4 to 6.6 s and in FG and EX the participants were allowed a time window of 2 s to catch the ball if tossed to. The fictive player smiled or frowned for 2.4 s (the latter together with a “drop” sound), depending on whether the participant caught the ball, to end the trial. The results showed that in FG participants successfully received the ball most of the time, with an average drop rate of 18.0 ± 21.6 (mean ± SD) %. Ball receiving trials occurred at trial 2, 6 (or 7), and 12 (or 13 or 14), depending on which fictive figure the subject tossed the ball to, in EX1 (exclusion session 1) and at trial 2, 8 (9), and 20 (21, 22) in EX2. In EX, the participants failed to catch the ball all the time, an outcome controlled by the program without participants’ knowledge. The two fictive players caught the ball successfully in all scenarios.
On average, a trial took 15.8 ± 4.8 s in OB, 14.0 ± 6.7 s in FG, and 15.2 ± 5.4 s in EX. With 16 (8x2) m per scenario, there were approximately 61 trials of observation in OB; 23 trials each of observation, ball tossed to, and from the participant in FG; and 57 trials of observation, and 6 trials each with ball tossed to and from the participant in EX. The small number of catch and loss trials were meant to elicit robust perception of exclusion in the EX. The program dictated that participants failed to catch to substantiate a rationale for exclusion by the fictive players in EX.
FMRI procedures and data analyses
Imaging was conducted with a 3-Tesla scanner (Siemens Trio, Erlangen, Germany), with scout scans, high-resolution MPRAGE, and BOLD scans acquired with multiband-multiplexed T2*-sensitive gradient-recalled, single-shot echo-planar imaging pulse sequence (iPat = 2, multiband = 4, TR = 1 s, TE = 31 ms, FoV = 192 mm, flip angle = 62°, matrix size = 96 x 96). Each volume consisted of 64 slices parallel to the bi-commissural plane (slice thickness 2 mm, no gap), and each BOLD run comprised 8 m or 480 volumes. Each scan comprised six 8-minute blood oxygenation level dependent (BOLD) runs of the Cyberball task.
All images were thoroughly inspected before pre-processing. BOLD data were analyzed with SPM12 (Wellcome Department of Imaging Neuroscience, University College London, U.K.). In the pre-processing of BOLD data, images of each participant were realigned (motion-corrected) and corrected for slice timing. A mean functional image volume was constructed for each participant for each run from the realigned image volumes. These mean images were co-registered with the high resolution structural image and then segmented for normalization to an MNI (Montreal Neurological Institute) EPI template with affine registration followed by nonlinear transformation (Friston et al., 1995; Ashburner & Friston, 1999). Finally, images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum. Images from the first five TRs at the beginning of each trial were discarded to ensure that the BOLD signals with steady-state equilibrium between radiofrequency pulsing and relaxation were included in the analyses.
A general linear model (GLM) was constructed for each individual subject, with the onsets of ball toss in each trial convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF and entered along with realignment parameters as regressors in the model (Friston et al., 1995). For OB, we identified a single event (ball toss between the two fictive players). For FG and EX, we distinguished three different events: ball toss from one to the other fictive player; ball toss to the participant; and ball toss initiated by the participant. Head motions in 6 dimensions were entered in the GLM. Serial autocorrelation was corrected by a first-degree autoregressive model and the data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts.
In the first-level analysis, we constructed for each individual subject contrasts of interest and the contrast (difference in β) images are used for group-level, random effects analysis (RFX; see Results). In RFX, we first employed a within-subject analysis of variance (ANOVA) to examine the session main effect (OB vs. FG vs. EX) of observation. Second, we examined the neural correlates of social interaction (tossing or receiving) versus observation by contrasting ball tossing and catching with observation trials. We employed a full factorial to examine the event main effect (tossing + receiving vs. observing), session main effect (FG vs. EX), and event × session interaction. Third, we examined the neural correlates of self-agency by comparing trials when participants tossed vs. received the ball. We employed a full factorial to examine the event main effect (tossing vs. receiving), session main effect (FG vs. EX), and event × session interaction. The contrast of tossing vs. receiving accounted for motor response (button press). However, participants successfully caught the ball most of the time in FG and failed to catch the ball all the time in EX. Thus, a contrast of tossing versus receiving may have involved emotion and other error-related processes. Thus, we also contrasted tossing versus observing in an additional model for comparison with tossing versus receiving and to identify the neural correlates of self-agency. All analyses were followed with planned comparisons to examine the sources of differences. All group analyses were evaluated at voxel p < 0.001, uncorrected, in combination with cluster p < 0.05 FWE, following current reporting standards (Poldrack et al., 2017). In addition, we investigated whether the differential regional responses to self-initiated actions between EX and FG may be related to individual differences in the RT differences in tossing or in tossing vs. receiving, and to the frequency at which participants tossed the ball back to the same fictive player, across EX and FG.
All data are available on the NIMH Data Archive (NDA) https://ndar.nih.gov/index.html.
Results
Behavioral findings
The reaction time (RT) of participants catching a ball was quantified by subtracting the time when the ball was thrown by a fellow player from the time when the participant pressed the button to catch the ball (Fig. 1b). The RT of participants throwing a ball was quantified by subtracting the time when the participant got hold of the ball from the time when the participant threw the ball (Fig. 1c). Both RTs were computed separately for the FG and EX session. We also quantified the frequency at which participants threw the ball to the same player from whom they just received the ball in a ratio of “same player/total throws” each for the FG and EX session (Fig. 1d).
Participants showed a significantly faster RT in throwing the ball during the FG compared with the EX session (1.60 ± 0.20 vs. 2.41 ± 0.31 s, t(23) = −4.56, p = 0.0001, Cohen’s d = 0.64, two-tailed paired-sample t test). In contrast, participants showed a significantly slower RT in receiving the ball during FG as compared to EX session (4.18 ± 0.07 vs. 4.02 ± 0.10 s, t(23) = 4.02, p = 0.0005, d = 0.37). The results showed no differences in the frequency with which participants threw to the same (from whom they just received the ball) versus the other player in the FG compared with EX session (same player/total throw ratio: 0.33 ± 0.03 vs. 0.39 ± 0.05, t(23) = −1.06, p = 0.30, d = 0.28, two-tailed paired-sample t test).
Imaging findings
OB, FG, and EX shared one single event: observing. FG and EX shared three distinct events: observing, receiving (R), and tossing (T). In the data analysis, we first examined regional activations to events that varied across OB, FG, and EX, or across FG and EX, with analyses of variance, followed by planned comparisons.
Observing peer interactions in OB, FG, and EX
The results of an analysis of variance (ANOVA) showed significant differences in activation of the precuneus (peak: x = −3, y = −52, z = 13; voxel Z = 4.1; 210 voxels, Supplementary Figure 1) during observations across the three scenarios, at voxel p < 0.001, uncorrected and cluster p < 0.05, FWE-corrected. Post-hoc comparisons of the β weights showed OB < FG (p = 0.023), OB > EX (p = 0.007), and FG > EX (p < 0.001), with the highest precuneus activity observed during FG and lowest during EX.
Interaction versus observation in FG and EX
We conducted a flexible factorial with scenarios (FG, EX) × events (observation or O vs. receiving or tossing ball or RT). The results showed higher activations across FG and EX in a wide swath of cortical and subcortical structures and lower activation in the paracentral lobule/posterior cingulate cortex and right somatomotor cortex during interactions with peers than during observation of peer interactions (FG_RT + EX_RT vs. FG_O + EX_O; Supplementary Figure 2a). Furthermore, there was a significant interaction effect (EX_RT > EX_O) > (FG_RT > FG_O) in bilateral superior frontal gyri (SFG), in the area of frontopolar cortex, right superior temporal sulcus (STS)/angular gyrus (AG), and right STS/middle temporal gyrus (MTG) (Supplementary Figure 2a; Supplementary Table 1). No other main or interaction effects yielded significant clusters. We identified the beta weights of individual events for each of these three clusters (Supplementary Figure 2b). In post-hoc comparisons, both STS/AG and STS/MTG showed higher activation during interaction than during observation in EX (all p's < 0.001) but not in FG (p = 0.558 and = 0.671, respectively). The SFG showed higher activation during interaction than during observation in EX (p = 0.018) but the opposite in FG (p = 0.039).
Self-initiated action in FG versus EX
The next analyses focused on identifying the neural correlates of the initiation of action during social interaction. To this end, we contrasted tossing (T) versus received (R) trials across FG and EX scenarios in a flexible factorial. The results showed distinct regional activation between ball tossing and receiving (Fig. 2a). Compared with R, T engaged bilateral middle/inferior frontal cortex and inferior parietal cortex, including angular and supramarginal gyri, superior temporal sulcus (STS)/middle temporal gyrus, precuneus/posterior cingulate cortex (PCu/PCC), occipital cortex and cerebellum. In contrast, R compared with T involved higher activation of bilateral somatomotor cortex, supplementary motor area, dorsal anterior cingulate cortex, thalamus, putamen, ventral striatum, caudate, and medial orbitofrontal cortex (Table 1). There also was a significant interaction effect in bilateral STS/angular gyrus (AG), PCu/PCC, and right inferior occipital/temporal gyrus (R IOTG). Figure 2b shows the beta weights of individual events for all four clusters, as identified from whole-brain analyses. In post-hoc comparisons, these four regions all showed higher activation during tossing than receiving in EX (all p's < 0.001) and in FG (all p's < 0.001 except for p < 0.05 for R ITG). For tossing trials, the R IOTG, L STS/AG, R STS/AG, and PCu/PCC showed higher activation in EX than FG (p < 0.001, p = 0.008, p < 0.001, and p = 0.017, respectively). For receiving trials, the R IOTG showed higher activation in FG than EX (p = 0.011), and the other clusters showed no differences between FG and EX.
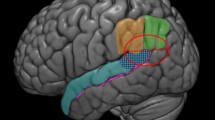
(a) Regional brain activations in a flexible factorial of scenarios (FG vs. EX) × events (tossing or T vs. receiving or R). Threshold: p = 0.001, uncorrected. For (FG_T + EX_T) vs. (FG_R + EX_R), shown in red are voxels of (FG_T + EX_T) > (FG_R + EX_R) and in blue are voxels of (FG_T + EX_T) < (FG_R + EX_R). Clusters with a peak voxel p<0.05 FWE are each shown in pink and light blue. Clusters meeting cluster p < 0.05, FWE-corrected are summarized in Table 1. (b) Histograms showing the beta weights (mean ± SD) of individual events for the four ROIs showing significant differences in (EX_T > EX_R) > (FG_T > FG_R). *p < 0.05; **p < 0.01; ***p < 0.001, paired t test. R: right; L: left, IOTG: inferior occipital/temporal gyrus; STS/AG: superior temporal sulcus/angular gyrus; PCu/PCC: precuneus/posterior cingulate cortex
By contrasting tossing and receiving trials, we accounted for motor actions. However, by experimental design, participants always failed to catch and may experience negative emotions and other error-related processes. Thus, we also compared tossing (T) and observing (O) trials across EX and FG. The results showed that, compared with O, T engaged extensive activations in cortical and subcortical regions, including a larger anterior cluster comprising inferior/middle/superior frontal gyri, dorsal anterior cingulate cortex, supplementary motor area, left thalamus, and orbitofrontal cortex; a large posterior cluster comprising the cerebellum, inferior parietal cortex, superior/middle/inferior temporal gyri, precuneus and posterior cingulate cortex; right thalamus; and right parahippocampal gyrus. The reverse contrast, O > T, engaged the paracentral lobule and right somatomotor cortex, medial orbitofrontal cortex, and right posterior insula. In scenario by event interaction, bilateral superior frontal gyrus (SFG), in the area of the frontopolar cortex, bilateral superior temporal sulcus (STS)/angular gyrus (AG), right middle temporal gyrus (MTG), bilateral inferior occipital gyrus (IOG) showed higher activation during T > O in EX compared with FG (Fig. 3a; Table 2). In post-hoc comparisons (Fig. 3b), the SFG showed higher activation during ball toss than observation in EX (p < 0.001), and the opposite in FG (p = 0.015); the right MTG, bilateral IOG, and left STS/AG all showed higher activation during ball toss than observation in EX (p < 0.001, p < 0.001, p < 0.001, and p < 0.001) and in FG (p = 0.003, p = 0.019, p = 0.030, and p = 0.005); the right STS/AG showed higher activation during ball toss than observation in EX (p < 0.001). For observation trials, the SFG, right STS/AG, bilateral IOG, and left STS/AG showed higher activation in FG than EX (p = 0.001, p = 0.005, p = 0.005, p = 0.011, and p < 0.001, respectively). For ball toss trials, the SFG, right MTG, right STS/AG, bilateral IOG, and left STS/AG all showed higher activation in EX than FG (p < 0.001, p < 0.001, p < 0.001, p < 0.001, p = 0.003, and p = 0.026, respectively).
(a) Regional brain activations in a flexible factorial of scenarios (FG vs. EX) × events (observing or O vs. tossing or T). Threshold: voxel p = 0.001, uncorrected. Red and blue color indicates clusters obtained of positive and negative contrast, respectively. Pink and light blue color show clusters with voxel p < 0.05 FWE. Yellow color shows voxels in (EX_T > EX_O) > (FG_T > FG_O) that overlapped those of (EX_T > EX_R) > (FG_T > FG_R) in Fig. 2a. Clusters meeting cluster p < 0.05, FWE-corrected are summarized in Table 2. (b) Histograms showing the beta weights (mean ± SD) of individual events for the six ROIs showing significant differences in (EX_T > EX_O) > (FG_T > FG_O). *p < 0.05; **p < 0.01; ***p < 0.001, paired t test. NS: nothing significant
We identified the regional responses to self-initiated action that differed between EX and FG by examining the voxels that overlapped between (EX_T > EX_R) > (FG_T > FG_R) and (EX_T > EX_O) > (FG_T > FG_O). The precuneus, bilateral AG, and right inferior temporal/occipital gyrus showed greater activation to tossing (vs. receiving and vs. observing) during EX than during FG (cluster p < 0.05 FWE). These three regions were combined for brain behavioral correlation analysis described in the next section. Furthermore, there were only a small number of tossing and receiving trials during EX. Thus, we plotted the time course of % signal change of these four regions of interest to show that the hemodynamic responses were properly modeled (Supplementary Figure 3).
Self-initiated action: brain behavior correlation
In behavioral performance, we showed that participants were faster in catching but slower in tossing the ball in the EX than in FG but were not different in the frequency of tossing the ball back to the same player from whom they just failed to catch the ball. By contrasting tossing versus receiving and versus observation trials across EX and FG, we identified regional activations to self-agency that increased in EX compared with FG. We tested whether the intersubject variation in these regional activities may relate to behavioral performance in two sets of linear regression. We computed the beta contrasts of regional activity for a single mask that comprised all four clusters (precuneus, bilateral angular gyri, and right inferior temporal/occipital gyrus; shown in yellow in Fig. 3a). The results were identical when the regressions were conducted separately for individual clusters; Supplementary Table 2). First, we correlated the RT difference (EX_T – EX_R) – (FG_T – FG_R) with the beta contrast (EX_T – EX_R) – (FG_T – FG_R) and the RT difference (EX_T – FG_T) with the beta contrast (EX_T – EX_O) – (FG_T – FG_O). The results did not show significant correlations (Fig. 4a and b). We next correlated the beta contrasts with the difference in the percentage of trials at which participants tossed the ball back to the same player in EX vs. FG. The results showed a significant correlation in both cases (all p < 0.001, Fig. 4c and d). The results remained significant even if we considered two data points from the contrast (EX_T – EX_R) – (FG_T – FG_R) and one data point from the contrast (EX_T – EX_O) – (FG_T – FG_O) as outliers (p = 0.003, R = 0.60; and p = 0.041, R = 0.43, respectively). Therefore, the latter finding is consistent with the proposition that increased regional responses to self-agency support the behavioral “burden” of social interactions during EX compared with FG.
Correlation of regional responses to self-agency to behavioral performance. The beta contrasts were derived of a single mask of all four clusters (precuneus, bilateral angular gyri, and right inferior temporal/occipital gyrus; yellow in Fig. 3A), showing significant differences in activity for (EX_T > EX_R) > (FG_T > FG_R) and (EX_T > EX_O) > (FG_T > FG_O). (a and b) The differences in regional activities did not correlate with RT difference between tossing and receiving across EX and FG or with RT difference in tossing between EX and FG. (c and d) The differences in regional activities correlated positively with the difference in the percentage of trials at which participants tossed the ball back to the same player across EX and FG
Discussion
Participants showed faster reaction time (RT) when receiving and slower RT when tossing ball during the scenario of social exclusion (EX) as compared with fair game (FG). The behavioral results suggested that participants were more eager to catch the ball but encumbered in throwing it back to fellow players when they were socially isolated. These behavioral findings support the thesis that initiating social interaction is harder during social isolation and that self-initiated action may represent a psychological dimension critical to social interaction. In imaging findings, we identified the neural processes of observation of peer interaction, social interaction, and self-initiated action that differ between EX and FG scenarios. Although participants did not show differences between EX and FG in the frequency at which they tossed the ball back to the same player, the difference was strongly correlated with the regional activities during self-initiated actions whether these activities were identified from contrasting tossing versus receiving or from tossing versus observing trials. Thus, increased regional responses support higher behavioral burden of self-initiated actions during EX compared with FG. We highlighted the major findings in the below.
Observation of social interactions
Observing the interaction of fellow players involved differences in precuneus activation across the three conditions. Compared with the scenario of OB, observations in FG involved higher activation of the ventral precuneus. In contrast, compared with both OB and FG, observations in EX involved lower activation or “deactivation” of the ventral precuneus. It is known that the precuneus is part of the default mode network (DMN), showing lower activation or “deactivation” when participants respond to environmental stimuli or engage in cognitive and affective tasks (Raichle et al., 2001). In particular, the precuneus comprised functional subdivisions, as demonstrated by the patterns of whole-brain functional connectivity, with the ventral precuneus most significantly connected with the DMN (Zhang & Li, 2012b). Further, earlier studies implicated the precuneus in the extent of effort involved in a cognitive task (Zhang & Li, 2010; Zhang & Li, 2012a). It thus appeared that sheer observation of peer interactions was affectively challenging when participants were excluded socially. Notably, the precuneus and posterior cingulate cortex were widely reported to be more active during social exclusion in earlier Cyberball studies (Rotge et al., 2014; Wang et al., 2017).
Social interactions versus observations
Interactions in the Cyberball task involved receiving the ball from and tossing the ball to fellow players. As expected, interaction compared with observation engaged many cortical and subcortical structures to support sensorimotor processing and decision making. On the other hand, observation as compared to interaction involved higher activation in the paracentral lobule (PCL)/posterior cingulate cortex (PCC) and right somatomotor cortex across FG and EX. Observation of movements alone or motor imagery was sufficient to engage the PCL/PCC (Cunnington, Windischberger, Robinson, & Moser, 2006; Munzert, Zentgraf, Stark, & Vaitl, 2008; Raos, Kilintari, & Savaki, 2014; Sacheli et al., 2017).
Furthermore, there was a significant interaction effect in bilateral superior frontal gyri (SFG), in the area of frontopolar cortex (FPC), right superior temporal sulcus (STS)/angular gyrus (AG), and right middle temporal gyrus (MTG). Both STS/AG and MTG showed higher activation during social interaction than during observation in EX but not in FG. The SFG showed higher activation during interaction than during observation in EX but the opposite in FG. The STS has been implicated in social interaction and psychological processes related to theory of mind in numerous studies (see Rushworth, Mars, & Sallet, 2013; Yang, Rosenblau, Keifer, & Pelphrey, 2015; Schurz, Tholen, Perner, Mars, & Sallet, 2017; Ninomiya, Noritake, Ullsperger, & Isoda, 2018 for a review). For instance, the STS along with temporal parietal junction and medial prefrontal cortex were consistently recruited for mental state reasoning, a process critical to social interaction (Koster-Hale et al., 2017). Although not typically highlighted in neuroimaging studies of social behavior, including those of the Cyberball task, the FPC is known to play an important role in “higher-level” decision-making processes, including the management of competing goals (Mansouri, Koechlin, Rosa, & Buckley, 2017; Peng, Steele, Becerra, & Borsook, 2017). Participants were perhaps negotiating the conflicting needs deciding which player to toss the ball to and, in EX, whether to toss the ball back to and appease the same player or to the other player in order to be fair. Thus, by distinguishing the individual events, we were able to characterize potentially critical activities not highlighted in previous Cyberball studies.
Self-initiated action: tossing versus catching and tossing versus observing
To the extent that it involves decision making about when and to whom to throw the ball, ball toss represents a self-initiated action and involves self-agency in the Cyberball task. We accounted for motor activities by contrasting ball tossing and receiving trials. On the other hand, participants missed the ball in all receiving trials during EX, and this contrast may reflect differences in emotional and other error-related responses. Thus, we also contrasted tossing with observing trials. The two contrasts involved largely similar brain regions. Tossing compared with receiving and observing both engaged activations of bilateral frontoparietal cortices and “deactivation” of the medial orbitofrontal cortex, supplementary motor area (SMA), and right-hemispheric somatomotor cortex (SMC). Importantly, there was a significant interaction effect. EX compared with FG engaged higher activity of the precuneus, bilateral angular gyri (AG), and right-hemispheric inferior temporal/occipital gyrus (ITG) to a greater extent during ball toss versus either ball catch or observation.
Self-initiated actions thus involved both shared and distinct neural processes during FG and EX. Tossing compared with receiving the ball required decision making, which engaged the frontal and parietal cortices. Decreased activation of the SMA and SMC may reflect the motor component of the decision making process (Toma et al., 2002) with tossing involving active decisions and more restrained motor response. Importantly, the precuneus, AG and ITG responded to self-initiated actions to a greater extent in EX compared with FG. These brain regions were commonly engaged during theory of mind and social emotional processing (Prochazkova et al., 2018; Thye, Ammons, Murdaugh, & Kana, 2018; Tsoi, Dungan, Chakroff, & Young, 2018), as well as during self-initiated actions in other behavioral contexts, as described earlier. The latter findings may reflect greater effort to engage in social interactions when participants were isolated. The results are in keeping with earlier studies of Cyberball task showing higher responses during social exclusion compared with inclusions (Rotge et al., 2014; Wang et al., 2017). These results are also broadly consistent with a recent study of the Cyberball task that reported increased regional connectivities within a mentalizing network during social exclusion relative to inclusion (Schmälzle et al., 2017).
Indeed, participants were slower in tossing the ball in EX than in FG. The behavioral burden, also reflected in the difference between EX and FG in the percentage of “toss to same” trials, were positively correlated with increased regional responses to self-initiated action. These findings together support the proposition that increases in regional responses to self-initiated action are needed, perhaps to overcome the psychological burden of peer interactions, when participants feel socially isolated.
A broader role of self-agency
The current study focused on the neural processes of self-initiated actions during social interactions in a relatively circumscribed context. These neural processes are reminiscent of those involved in self-agency and theory of mind processing. However, it is worth noting that we did not evaluate the sense of self-involvement and participants were instructed to catch and toss the ball—to engage in the interaction. Thus, to the extent that ball tosses are dictated by the behavioral task, these neural processes may not speak directly to self-agency. Furthermore, self-agency may play a role in decision making more broadly in and beyond social interactions; e.g., in determining individual differences in control-averse behavior (Rudorf et al., 2018), in modulating choice behavior when unexpected outcomes arise from errors in action (Parvin, McDougle, Taylor, & Ivry, 2018), and in mentalizing to assign third-party punishment (Ginther et al., 2016). The current findings may add to this literature and facilitate research of the influences of social exclusion on many other decision making processes that involve social interactions.
Conclusions, limitations of the study, and potential clinical implications
The current findings are the first to distinguish self-initiated actions by characterizing the behavioral and neural correlates of ball tossing versus receiving in the Cyberball task. The findings suggest that social exclusions encumbered self-initiated actions, which engaged the precuneus, angular gyrus, and posterior superior temporal cortex, regions widely implicated in social emotional processing.
A few limitations need to be considered. First, although the behavioral and imaging findings were all reported with a corrected threshold, the sample size is relatively small and the findings would require replication. Second, at debriefing participants expressed frustration that they failed to catch the ball despite trying and received fewer throws in the exclusion sessions. A few subjects mentioned that later in the task they wondered whether it was just a computer game. Thus, although an earlier behavioral study showed that social exclusion elicited negative cognitive and affective responses even if the participants were told that they were interacting with a computerized game (Zadro, Williams, & Richardson, 2004), the differences in how simulated and real-life social interactions impact our behavior remain to be clarified.
Finally, although the study focused on healthy populations, the findings may have implications for future research of the etiologies of clinical conditions that involve difficulties in social communications or social anxiety. Social cognition represents an important research domain of many mental conditions, including social anxiety disorder (Gur & Gur, 2016; Silk et al., 2013; Rudolph, Miernicki, Troop-Gordon, Davis, & Telzer, 2016; Heeren et al., 2017). The findings characterizing cerebral responses to self-initiated actions during social exclusions may help to unravel the neural markers of these social behavioral disorders.
References
Ashburner, J., & Friston, K. J. (1999). Nonlinear spatial normalization using basis functions. Human Brain Mapping, 7, 254–266.
Atkinson, A. P., & Adolphs, R. (2011). The neuropsychology of face perception: Beyond simple dissociations and functional selectivity. Philosophical Transactions of the Royal Society B, 366, 1726–1738.
Bauminger, N. (2002). The facilitation of social-emotional understanding and social interaction in high-functioning children with autism: Intervention outcomes. Journal of Autism and Developmental Disorders, 32, 283–298.
Bishop, S., Gahagan, S., & Lord, C. (2007). Re-examining the core features of autism: A comparison of autism spectrum disorder and fetal alcohol spectrum disorder. Journal of Child Psychology and Psychiatry, 48, 1111–1121.
Bolling, D. Z., Pitskel, N. B., Deen, B., Crowley, M. J., McPartland, J. C., Mayes, L. C., & Pelphrey, K. A. (2011). Dissociable brain mechanisms for processing social exclusion and rule violation. Neuroimage, 54, 2462–2471.
Brown, R. C., Plener, P. L., Groen, G., Neff, D., Bonenberger, M., & Abler, B. (2017). Differential neural processing of social exclusion and inclusion in adolescents with non-suicidal self-injury and young adults with borderline personality disorder. Frontiers in Psychiatry, 8.
Carter, A. S., Davis, N. O., Klin, A., & Volkmar, F. R. (2005). Social development in autism. In F. R. Volkmar, R. Paul, A. Klin, & D. Cohen (Eds.), Handbook of autism and pervasive developmental disorders: Vol. 1. Diagnosis, development, neurobiology, and behavior. Hoboken, NJ: John Wiley & Sons.
Cunnington, R., Windischberger, C., Robinson, S., & Moser, E. (2006). The selection of intended actions and the observation of others’ actions: A time-resolved fMRI study. Neuroimage, 29, 1294–1302.
Davis, F. C., Neta, M., Kim, M. J., Moran, J. M., & Whalen, P. J. (2016). Interpreting ambiguous social cues in unpredictable contexts. Social Cognitive and Affective Neuroscience, 11, 775–782.
de Bezenac, C. E., Sluming, V., Gouws, A., & Corcoran, R. (2016). Neural response to modulating the probability that actions of self or other result in auditory tones: A parametric fMRI study into causal ambiguity. Biological Psychology, 119, 64–78.
DeWall, C. N., MacDonald, G., Webster, G. D., Masten, C. L., Baumeister, R. F., Powell, C., . . . Eisenberger, N. I. (2010). Acetaminophen reduces social pain: Behavioral and neural evidence. Psychological Science, 21, 931-937.
Domsalla, M., Koppe, G., Niedtfeld, I., Vollstädt-Klein, S., Schmahl, C., Bohus, M., & Lis, S. (2013). Cerebral processing of social rejection in patients with borderline personality disorder. Social Cognitive and Affective Neuroscience, 9, 1789–1797.
Eisenberger, N. I., Lieberman, M. D., & Williams, K. D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302, 290–292.
Emmerling, F., Schuhmann, T., Lobbestael, J., Arntz, A., Brugman, S., & Sack, A. T. (2016). The role of the insular cortex in retaliation. Plos One, 11.
Friston, K., Holmes, A. P., Worsley, K. J., Poline, J. B., Frith, C. D., & Frackowiak, R. (1995). Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping, 2, 189–210.
Fukushima, H., Goto, Y., Maeda, T., Kato, M., & Umeda, S. (2013). Neural substrates for judgment of self-agency in ambiguous situations. Plos One, 8.
Fusar-Poli, P., Placentino, A., Carletti, F., Landi, P., Allen, P., Surguladze, S., . . . Politi, P. (2009). Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience, 34, 418-432.
Ginther, M. R., Bonnie, R. J., Hoffman, M. B., Shen, F. X., Simons, K. W., Jones, O. D., & Marois, R. (2016). Parsing the behavioral and brain mechanisms of third-party punishment. Journal of Neuroscience, 36, 9420–9434.
Gonzalez, M. Z., Beckes, L., Chango, J., Allen, J. P., & Coan, J. A. (2014). Adolescent neighborhood quality predicts adult dACC response to social exclusion. Social Cognitive and Affective Neuroscience, 10, 921–928.
Gur, R. C., & Gur, R. E. (2016). Social cognition as an RDoC domain. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171, 132–141.
Hartgerink, C. H. J., van Beest, I., Wicherts, J. M., & Williams, K. D. (2015). The ordinal effects of ostracism: A meta-analysis of 120 cyberball studies. Plos One, 10.
Heeren, A., Dricot, L., Billieux, J., Philippot, P., Grynberg, D., De Timary, P., & Maurage, P. (2017). Correlates of social exclusion in social anxiety disorder: An fMRI study. Scientific Reports, 7, 260.
Jankowski, K. F., Batres, J., Scott, H., Smyda, G., Pfeifer, J. H., & Quevedo, K. (2018). Feeling left out: Depressed adolescents may atypically recruit emotional salience and regulation networks during social exclusion. Social Cognitive and Affective Neuroscience, 13, 863–876.
Klapwijk, E. T., Lelieveld, G. J., Aghajani, M., Boon, A. E., van der Wee, N. J. A., Popma, A., . . . Colins, O. F. (2016). Fairness decisions in response to emotions: A functional MRI study among criminal justice-involved boys with conduct disorder. Social Cognitive and Affective Neuroscience, 11, 674-682.
Koban, L., & Pourtois, G. (2014). Brain systems underlying the affective and social monitoring of actions: An integrative review. Neuroscience & Biobehavioral Reviews, 46, 71–84.
Koster-Hale, J., Richardson, H., Velez, N., Asaba, M., Young, L., & Saxe, R. (2017). Mentalizing regions represent distributed, continuous, and abstract dimensions of others’ beliefs. Neuroimage, 161, 9–18.
Kross, E., Egner, T., Ochsner, K., Hirsch, J., & Downey, G. (2007). Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience, 19, 945–956.
Kumar, P., Waiter, G. D., Dubois, M., Milders, M., Reid, I., & Steele, J. D. (2017). Increased neural response to social rejection in major depression. Depression and Anxiety, 34, 1049–1056.
Lieberman, M. D., & Eisenberger, N. I. (2009). Neuroscience pains and pleasures of social life. Science, 323, 890–891.
Maner, J. K., DeWall, C. N., Baumeister, R. F., & Schaller, M. (2007). Does social exclusion motivate interpersonal reconnection? Resolving the “porcupine problem”. Journal of Personality and Social Psychology, 92, 42.
Mansouri, F. A., Koechlin, E., Rosa, M. G., & Buckley, M. J. (2017). Managing competing goals—A key role for the frontopolar cortex. Nature Reviews Neuroscience, 18, 645.
Martelli, A. M., Chester, D. S., Warren Brown, K., Eisenberger, N. I., & Nathan DeWall, C. (2018). When less is more: mindfulness predicts adaptive affective responding to rejection via reduced prefrontal recruitment. Social Cognitive and Affective Neuroscience.
Mattick, R. P., & Clarke, J. C. (1998). Development and validation of measures of social phobia scrutiny fear and social interaction anxiety1. Behaviour Research and Therapy, 36, 455–470.
Maurer, C. W., LaFaver, K., Ameli, R., Epstein, S. A., Hallett, M., & Horovitz, S. G. (2016). Impaired self-agency in functional movement disorders a resting-state fMRI study. Neurology, 87, 564–570.
Moor, B. G., Guroglu, B., Op de Macks, Z. A., Rombouts, S. A. R. B., Van der Molen, M. W., & Crone, E. A. (2012). Social exclusion and punishment of excluders: Neural correlates and developmental trajectories. Neuroimage, 59, 708–717.
Munzert, J., Zentgraf, K., Stark, R., & Vaitl, D. (2008). Neural activation in cognitive motor processes: Comparing motor imagery and observation of gymnastic movements. Experimental Brain Research, 188, 437–444.
Nahab, F. B., Kundu, P., Gallea, C., Kakareka, J., Pursley, R., Pohida, T., . . . Hallett, M. (2011). The neural processes underlying self-agency. Cerebral Cortex, 21, 48-55.
Nakagawa, S., Takeuchi, H., Taki, Y., Nouchi, R., Sekiguchi, A., Kotozaki, Y., . . . Kawashima, R. (2017). The anterior midcingulate cortex as a neural node underlying hostility in young adults. Brain Structure & Function, 222, 61-70.
Ninomiya, T., Noritake, A., Ullsperger, M., & Isoda, M. (2018). Performance monitoring in the medial frontal cortex and related neural networks: From monitoring self actions to understanding others’ actions. Neuroscience Research.
Parvin, D. E., McDougle, S. D., Taylor, J. A., & Ivry, R. B. (2018). Credit assignment in a motor decision making task is influenced by agency and not sensorimotor prediction errors. Journal of Neuroscience, 3601–3617.
Peng, K., Steele, S. C., Becerra, L., & Borsook, D. (2017). Brodmann area 10: Collating, integrating and high level processing of nociception and pain. Progress in Neurobiology.
Poldrack, R. A., Baker, C. I., Durnez, J., Gorgolewski, K. J., Matthews, P. M., Munafo, M. R., . . . Yarkoni, T. (2017). Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nature Reviews. Neuroscience, 18, 115-126.
Prochazkova, E., Prochazkova, L., Giffin, M. R., Scholte, H. S., De Dreu, C. K., & Kret, M. E. (2018). Pupil mimicry promotes trust through the theory-of-mind network. Proceedings of the National Academy of Sciences, 201803916.
Radke, S., Seidel, E. M., Eickhoff, S. B., Gur, R. C., Schneider, F., Habel, U., & Derntl, B. (2016). When opportunity meets motivation: Neural engagement during social approach is linked to high approach motivation. Neuroimage, 127, 267–276.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98, 676–682.
Raos, V., Kilintari, M., & Savaki, H. E. (2014). Viewing a forelimb induces widespread cortical activations. Neuroimage, 89, 122–142.
Redcay, E., Kleiner, M., & Saxe, R. (2012). Look at this: The neural correlates of initiating and responding to bids for joint attention. Frontiers in Human Neuroscience, 6, 169.
Renes, R. A., van Haren, N. E. M., Aarts, H., & Vink, M. (2015). An exploratory fMRI study into inferences of self-agency. Social Cognitive and Affective Neuroscience, 10, 708–712.
Rotge, J.-Y., Lemogne, C., Hinfray, S., Huguet, P., Grynszpan, O., Tartour, E., . . . Fossati, P. (2014). A meta-analysis of the anterior cingulate contribution to social pain. Social Cognitive and Affective Neuroscience, 10, 19-27.
Rudolph, K. D., Miernicki, M. E., Troop-Gordon, W., Davis, M. M., & Telzer, E. H. (2016). Adding insult to injury: Neural sensitivity to social exclusion is associated with internalizing symptoms in chronically peer-victimized girls. Social Cognitive and Affective Neuroscience, 11, 829–842.
Rudorf, S., Schmelz, K., Baumgartner, T., Wiest, R., Fischbacher, U., & Knoch, D. (2018). Neural mechanisms underlying individual differences in control-averse behavior. Journal of Neuroscience, 0047–0018.
Rushworth, M. F., Mars, R. B., & Sallet, J. (2013). Are there specialized circuits for social cognition and are they unique to humans? Current Opinion in Neurobiology, 23, 436–442.
Ruys, K. I., & Aarts, H. (2012). I didn't mean to hurt you! Unconscious origins of experienced self-agency over others' emotions. Emotion, 12, 132.
Sabatinelli, D., Fortune, E. E., Li, Q. Y., Siddiqui, A., Krafft, C., Oliver, W. T., . . . Jeffries, J. (2011). Emotional perception: Meta-analyses of face and natural scene processing. Neuroimage, 54, 2524-2533.
Sacheli, L. M., Zapparoli, L., De Santis, C., Preti, M., Pelosi, C., Ursino, N., . . . Paulesu, E. (2017). Mental steps: Differential activation of internal pacemakers in motor imagery and in mental imitation of gait. Human Brain Mapping, 38, 5195-5216.
Schmälzle, R., O’Donnell, M. B., Garcia, J. O., Cascio, C. N., Bayer, J., Bassett, D. S., . . . Falk, E. B. (2017). Brain connectivity dynamics during social interaction reflect social network structure. Proceedings of the National Academy of Sciences, 114, 5153-5158.
Schurz, M., Tholen, M. G., Perner, J., Mars, R. B., & Sallet, J. (2017). Specifying the brain anatomy underlying temporo-parietal junction activations for theory of mind: A review using probabilistic atlases from different imaging modalities. Human Brain Mapping, 38, 4788–4805.
Sebastian, C. L., Tan, G. C. Y., Roiser, J. P., Viding, E., Dumontheil, I., & Blakemore, S. J. (2011). Developmental influences on the neural bases of responses to social rejection: Implications of social neuroscience for education. Neuroimage, 57, 686–694.
Silk, J. S., Siegle, G. J., Lee, K. H., Nelson, E. E., Stroud, L. R., & Dahl, R. E. (2013). Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience, 9, 1798–1807.
Spaniel, F., Tintera, J., Rydlo, J., Ibrahim, I., Kasparek, T., Horacek, J., . . . , Hajek, T. (2016). Altered neural correlate of the self-agency experience in first-episode schizophrenia-spectrum patients: An fMRI study. Schizophrenia Bulletin, 42, 916-925.
Sperduti, M., Delaveau, P., Fossati, P., & Nadel, J. (2011). Different brain structures related to self- and external-agency attribution: A brief review and meta-analysis. Brain Structure & Function, 216, 151–157.
Srinivasan, R., Golomb, J. D., & Martinez, A. M. (2016). A neural basis of facial action recognition in humans. Journal of Neuroscience, 36, 4434–4442.
Thye, M. D., Ammons, C. J., Murdaugh, D. L., & Kana, R. K. (2018). Differential recruitment of theory of mind brain network across three tasks: An independent component analysis. Behavioural Brain Research, 347, 385–393.
Toma, K., Mima, T., Matsuoka, T., Gerloff, C., Ohnishi, T., Koshy, B., . . . Hallett, M. (2002). Movement rate effect on activation and functional coupling of motor cortical areas. Journal of Neurophysiology, 88, 3377-3385.
Tsoi, L., Dungan, J. A., Chakroff, A., & Young, L. L. (2018). Neural substrates for moral judgments of psychological versus physical harm. Social Cognitive and Affective Neuroscience, 13, 460–470.
van der Meulen, M., Steinbeis, N., Achterberg, M., Bilo, E., van den Bulk, B. G., van IJendoorn, M. H., & Crone, E. A. (2017). The neural correlates of dealing with social exclusion in childhood. Neuropsychologia, 103, 29–37.
Voss, M., Chambon, V., Wenke, D., Kuhn, S., & Haggard, P. (2017). In and out of control: Brain mechanisms linking fluency of action selection to self-agency in patients with schizophrenia. Brain, 140, 2226–2239.
Wang, H. Y., Braun, C., & Enck, P. (2017). How the brain reacts to social stress (exclusion) - A scoping review. Neuroscience and Biobehavioral Reviews, 80, 80–88.
Wasylyshyn, N., Hemenway Falk, B., Garcia, J. O., Cascio, C. N., O’donnell, M. B., Bingham, C. R., . . . Falk, E. B. (2018). Global brain dynamics during social exclusion predict subsequent behavioral conformity. Social Cognitive and Affective Neuroscience, 13, 182-191.
Weiss, C., Herwig, A., & Schütz-Bosbach, S. (2011). The self in social interactions: Sensory attenuation of auditory action effects is stronger in interactions with others. PLoS One, 6, e22723.
Whittle, S., Liu, K., Bastin, C., Harrison, B. J., & Davey, C. G. (2016). Neurodevelopmental correlates of proneness to guilt and shame in adolescence and early adulthood. Developmental Cognitive Neuroscience, 19, 51–57.
Williams, K. D., & Jarvis, B. (2006). Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behavior Research Methods, 38, 174–180.
Yang, D. Y.-J., Rosenblau, G., Keifer, C., & Pelphrey, K. A. (2015). An integrative neural model of social perception, action observation, and theory of mind. Neuroscience & Biobehavioral Reviews, 51, 263–275.
Zadro, L., Williams, K. D., & Richardson, R. (2004). How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. Journal of Experimental Social Psychology, 40, 560–567.
Zahn, R., Moll, J., Paiva, M., Garrido, G., Krueger, F., Huey, E. D., & Grafman, J. (2008). The neural basis of human social values: evidence from functional MRI. Cerebral Cortex, 19, 276–283.
Zhang, S., & Li, C.S. (2010). A neural measure of behavioral engagement: task-residual low-frequencyblood oxygenation level-dependent activity in the precuneus. Neuroimage, 49, 1911–1918.
Zhang, S., & Li, C.S. (2012a). Task-related, low-frequency task-residual, and resting state activity in thedefault mode network brain regions. Frontiers in Psychology, 3, 172.
Zhang, S., & Li, C. R. (2012b). Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage, 59, 3548–3562.
Acknowledgments
The study was supported by NIH grant R21MH113134. The funding agencies were otherwise not involved in data collection or analysis, or in the decision to publish these results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We have no conflicts of interest in the current work.
Open practices statement
The data and materials for the experiments have been deposited and are available at the NIMH Data Archive (NDA) https://ndar.nih.gov/index.html. The experiment was not preregistered.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1884 kb)
Rights and permissions
About this article
Cite this article
Wang, W., Zhornitsky, S., Li, C.SP. et al. Cerebral responses to self-initiated action during social interactions. Cogn Affect Behav Neurosci 19, 1521–1535 (2019). https://doi.org/10.3758/s13415-019-00711-5
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-019-00711-5