Abstract
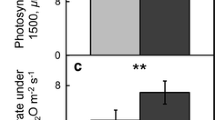
Plants can change leaf forms, adjusting light conditions on their adaxial and abaxial surfaces, to adapt to light environments and enhance their light use efficiencies. The difference between photosynthesis on the two leaf sides (dorsoventral asymmetry) is an important factor that affects light use efficiency. However, photosynthetic dorsoventral asymmetry is rarely compared under direct and diffuse light conditions. To estimate the impacts of recently reported alterations in direct and diffuse light in the sky radiation on plant carbon assimilation, variations in morphology between the two leaf sides in tobacco (Nicotiana tabacum L.) were investigated, and the dorsoventral responses of photosynthesis to illuminating directions were compared in direct and diffuse light. Dorsoventral asymmetry was reflected in stomatal densities, anatomic structures, and photochemical traits, which caused markedly different photosynthetic rates as well as stomatal conductances both in direct and diffuse light. However, the degree of photosynthetic asymmetry was weakened in diffuse light. The diffuse light caused a greater stomatal conductance on the abaxial side than direct light, which resulted in reduced photosynthetic asymmetry. In addition, the photosynthetic dorsoventral asymmetry could be affected by the photosynthetic photon flux density. These results contribute to understanding the dorsoventral regulation of photosynthesis in bifacial leaves, and provide a reference for breeding to cope with the increase in the proportion of diffuse light in the future.







Similar content being viewed by others
References
Bone RA, Lee DW, Norman JM (1985) Epidermal cells functioning as lenses in leaves of tropical rain-forest shade plants. Appl Opt 24:1408–1412
Bornman JF, Vogelmann TC, Martin G (1991) Measurement of chlorophyll fluorescence within leaves using a fibreoptic microprobe. Plant Cell Environ 14:719–725
Brodersen CR, Vogelmann TC (2007) Do epidermal lens cells facilitate the absorptance of diffuse light? Am J Bot 94:1061–1066
Brodersen CR, Vogelmann TC (2010) Do changes in light direction affect absorption profiles in leaves? Funct Plant Biol 37:403–412
Brodersen CR, Vogelmann TC, Williams WE, Gorton HL (2008) A new paradigm in leaf-level photosynthesis: direct and diffuse lights are not equal. Plant Cell Environ 31:159–164
Chen Y, Murchie EH, Hubbart S, Horton P, Peng S (2003) Effects of season-dependent irradiance levels and nitrogen-deficiency on photosynthesis and photoinhibition in field-grown rice (Oryza sativa). Physiol Plant 117:343–351
Chen Q, Xie Q, Gao J, Wang W, Sun B, Liu B, Zhu H, Peng H, Zhao H, Liu C, Wang J, Zhang J, Zhang G, Zhang Z (2015) Characterization of rolled and erect leaf in regulating leave morphology in rice. J Exp Bot 66:6047–6058
Coupe SA, Palmer BG, Lake JA, Overy SA, Oxborough K, Woodward FI, Gray JE, Quick WP (2006) Systemic signalling of environmental cues in Arabidopsis leaves. J Exp Bot 57:329–341
de Visser PHB, Sarlikioti V, Marcelis LFM, Buck-Sorlin GH (2011) How plant architecture affects light absorption and photosynthesis in tomato: towards an ideotype for plant architecture using a functional-structural plant model. Ann Bot 108:1065–1073
Delucia EH, Shenoi HD, Naidu SL, Day TA (1991) Photosynthetic symmetry of sun and shade leaves of different orientations. Oecologia 87:51–57
Earles JM, Théroux-Rancourt G, Gilbert ME, McElrone AJ, Brodersen CR (2017) Excess diffuse light absorption in upper mesophyll limits CO2 drawdown and depresses photosynthesis. Plant Physiol 174:1082–1096
Evans JR, Jakobsen I, Ögren E (1993) Photosynthetic light-response curves. Planta 189:191–200
Ezhova E, Ylivinkka I, Kuusk J, Komsaare K, Vana M, Krasnova A, Noe S, Arshinov M, Belan B, Park SB, Lavrič JV, Heimann M, Petäjä T, Vesala T, Mammarella I, Kolari P, Bäck J, Rannik Ü, Kerminen VM, Kulmala M (2018) Direct effect of aerosols on solar radiation and gross primary production in boreal and hemiboreal forests. Atmos Chem Phys 18:17863–17881
Farquhar GD, Roderick ML (2003) Pinatubo, diffuse light, and the carbon cycle. Science 299:1997–1998
Gilmore AM (1997) Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant 99:197–209
Gorton HL, Brodersen CR, Williams WE, Vogelmann TC (2010) Measurement of the optical properties of leaves under diffuse light. Photochem Photobiol 86:1076–1083
Haupt W, Scheuerlein R (1990) Chloroplast movement. Plant Cell Environ 13:595–614
Higa T, Wada M (2016) Chloroplast avoidance movement is not functional in plants grown under strong sunlight. Plant Cell Environ 39:871–882
Hughes NM, Carpenter KL, Cook DK, Keidel TS, Miller CN, Neal JL, Sanchez A, Smith WK (2015) Effects of cumulus clouds on microclimate and shoot-level photosynthetic gas exchange in Picea engelmannii and Abies lasiocarpa at treeline, Medicine Bow Mountains, Wyoming, USA. Agric For Meteorol 201:26–37
Husbands AY, Benkovics AH, Nogueira FTS, Lodha M, Timmermans MCP (2015) The ASYMMETRIC LEAVES complex employs multiple modes of regulation to affect adaxial-abaxial patterning and leaf complexity. Plant Cell 27:3321–3335
Jacquemoud S, Baret F (1990) PROSPECT: a model of leaf optical properties spectra. Remote Sensing Environ 34:75–91
Jiang C-D, Wang X, Gao H-Y, Shi L, Chow WS (2011) Systemic regulation of leaf anatomical structure, photosynthetic performance, and high-light tolerance in Sorghum. Plant Physiol 155:1416–1424
Kanniah KD, Beringer J, North P, Hutley L (2012) Control of atmospheric particles on diffuse radiation and terrestrial plant productivity: a review. Progress Phys Geograph 36:209–237
Kumagai E, Hamaoka N, Araki T, Ueno O (2014) Dorsoventral asymmetry of photosynthesis and photoinhibition in flag leaves of two rice cultivars that differ in nitrogen response and leaf angle. Physiol Plant 151:533–543
Lawson T (2009) Guard cell photosynthesis and stomatal function. New Phytol 181:13–34
Li T, Yang Q (2015) Advantages of diffuse light for horticultural production and perspectives for further research. Front Plant Sci 6:704
Long SP, Farage PK, Bolharnordenkampf HR, Rohrhofer U (1989) Separating the contribution of the upper and lower mesophyll to photosynthesis in Zea mays L. leaves. Planta 177:207–216
Mantilla-Perez MB, Salas Fernandez MG (2017) Differential manipulation of leaf angle throughout the canopy: current status and prospects. J Exp Bot 68:5699–5717
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668
Mercado LM, Bellouin N, Sitch S, Boucher O, Huntingford C, Wild M, Cox PM (2009) Impact of changes in diffuse radiation on the global land carbon sink. Nature 458:1014–1087
Moon J, Hake S (2011) How a leaf gets its shape. Curr Opin Plant Biol 14:24–30
Moss DN (1964) Optimum lighting of leaves. Crop Sci 4:131–136
Mott KA, Peak D (2018) Effects of the mesophyll on stomatal responses in amphistomatous leaves. Plant Cell Environ 41:2835–2843
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927
Oguchi R, Douwstra P, Fujita T, Chow WS, Terashima I (2011) Intra-leaf gradients of photoinhibition induced by different color lights: implications for the dual mechanisms of photoinhibition and for the application of conventional chlorophyll fluorometers. New Phytol 191:146–159
Oguchi R, Onoda Y, Terashima I, Tholen D (2018) Leaf anatomy and function. In: Adams WW, Terashima I (eds) The leaf: a platform for performing photosynthesis. Springer International Publishing, Cham, pp 97–139
Qian T, Elings A, Dieleman JA, Gort G, Marcelis LFM (2012) Estimation of photosynthesis parameters for a modified Farquhar–von Caemmerer-Berry model using simultaneous estimation method and nonlinear mixed effects model. Environ Exp Bot 82:66–73
Rap A, Scott CE, Reddington CL, Mercado L, Ellis RJ, Garraway S, Evans MJ, Beerling DJ, MacKenzie AR, Hewitt CN, Spracklen DV (2018) Enhanced global primary production by biogenic aerosol via diffuse radiation fertilization. Nat Geosci 11:640–644
Reinhardt K, Smith WK (2008) Impacts of cloud immersion on microclimate, photosynthesis and water relations of Abies fraseri (Pursh.) Poiret in a temperate mountain cloud forest. Oecologia 158:229–238
Reinhardt K, Smith WK (2016) Chlorophyll fluorescence and photosynthetic gas exchange under direct versus diffuse light in evergreen conifer (Picea pungens) shoots and broadleaf shrub (Rhododendron ponticum) leaves. Plant Ecol 217:443–450
Reinhardt K, Smith WK, Carter GA (2010) Clouds and cloud immersion alter photosynthetic light quality in a temperate mountain cloud forest. Botany-Botanique 88:462–470
Richardson F, Brodribb TJ, Jordan GJ (2017) Amphistomatic leaf surfaces independently regulate gas exchange in response to variations in evaporative demand. Tree Physiol 37:869–878
Shimazaki KI, Doi M, Assmann SM, Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58:219–247
Smith WK, Vogelmann TC, DeLucia EH, Bell DT, Shepherd KA (1997) Leaf form and photosynthesis. Bioscience 47:785–793
Soares AS, Driscoll SP, Olmos E, Harbinson J, Arrabaca MC, Foyer CH (2008) Adaxial/abaxial specification in the regulation of photosynthesis and stomatal opening with respect to light orientation and growth with CO2 enrichment in the C4 species Paspalum dilatatum. New Phytol 177:186–198
Soares-Cordeiro AS, Driscoll SP, Pellny TK, Olmos E, Arrabaca MC, Foyer CH (2009) Variations in the dorso-ventral organization of leaf structure and Kranz anatomy coordinate the control of photosynthesis and associated signalling at the whole leaf level in monocotyledonous species. Plant Cell Environ 32:1833–1844
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the Chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Netherlands, pp 321–362
Sun J, Nishio JN, Vogelmann TC (1996) High-light effects on CO2 fixation gradients across leaves. Plant Cell Environ 19:1261–1271
Terashima I, Inoue Y (1984) Comparative photosynthetic properties of palisade tissue chloroplasts and spongy tissue chloroplasts of Camellia japonica L.: functional adjustment of the photosynthetic apparatus to light environment within a leaf. Plant Cell Physiol 25:555–563
Terashima I, Saeki T (1983) Light environment within a leaf I. Optical properties of paradermal sections of Camellia leaves with special reference to differences in the optical properties of palisade and spongy tissues. Plant Cell Physiol 24:1493–1501
Thain JF (1983) Curvature correction factors in the measurement of cell-surface areas in plant tissues. J Exp Bot 34:87–94
Thornley JH (1976) Mathematical models in plant physiology. Academic Press (Inc.) London, Ltd., London
Tsimilli-Michael M, Eggenberg P, Biro B, Köves-Pechy K, Vörös I, Strasser RJ (2000) Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient O-J-I-P. App Soil Ecol 15:169–182
Urban O, Klem K, Ac A, Havrankova K, Holisova P, Navratil M, Zitova M, Kozlova K, Pokorny R, Sprtova M, Tomaskova I, Spunda V, Grace J (2012) Impact of clear and cloudy sky conditions on the vertical distribution of photosynthetic CO2 uptake within a spruce canopy. Funct Ecol 26:46–55
Van Heerden PDR, Strasser RJ, Kruger GH (2004) Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiol Plant 121:239–249
Vogelmann TC, Gorton HL (2014) Leaf: light capture in the photosynthetic organ. In: Hohmann-Marriott MF (ed) The structural basis of biological energy generation. Springer, Netherlands, pp 363–377
Vogelmann TC, Bornman JF, Yates DJ (1996) Focusing of light by leaf epidermal cells. Physiol Plant 98:43–56
Wang Y, Noguchi K, Terashima I (2008) Distinct light responses of the adaxial and abaxial stomata in intact leaves of Helianthus annuus L. Plant Cell Environ 31:1307–1316
Williams M, Rastetter EB, Van der Pol L, Shaver GR (2014) Arctic canopy photosynthetic efficiency enhanced under diffuse light, linked to a reduction in the fraction of the canopy in deep shade. New Phytol 202:1266–1276
Wu B-J, Chow WS, Liu Y-J, Shi L, Jiang C-D (2014) Effects of stomatal development on stomatal conductance and on stomatal limitation of photosynthesis in Syringa oblata and Euonymus japonicus Thunb. Plant Sci 229:23–31
Yaacob MB (1982) Quantitative inheritance of leaf shape characters in tobacco (Nicotiana tabacum L.): a thesis presented in partial fulfilment of the requirements for the degree of Master of Agricultural Science in Plant Science at Massey University, Palmerston North, New Zealand. Dissertation, Massey University
Zhang G-H, Xu Q, Zhu X-D, Qian Q, Xue H-W (2009) SHALLOT-LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. Plant Cell 21:719–735
Zou LP, Sun XH, Zhang ZG, Liu P, Wu JX, Tian CJ, Qiu JL, Lu TG (2011) Leaf rolling controlled by the homeodomain leucine zipper class IV gene Roc5 in rice. Plant Physiol 156:1589–1602
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Yan, H., Wu, B. et al. Dorsoventral photosynthetic asymmetry of tobacco leaves in response to direct and diffuse light. J Plant Res 133, 35–48 (2020). https://doi.org/10.1007/s10265-019-01151-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-019-01151-5