Abstract
One of the most significant pathologies of Alzheimer’s disease (AD), an irreversible and progressive neurodegenerative disease that causes cognitive impairment, is the neuroinflammation facilitating the accumulation of amyloid-β (Aβ) peptide. Hence, the inhibition of abnormal neuroinflammatory response is considered a promising therapeutic approach for AD. Picrorhiza kurroa Bentham, Scrophulariae (PK) is a medicinal herb that has been traditionally used for the treatment of various diseases, including inflammation. This study aims to report the significance of PK treatment in markedly improving spatial learning memory and dramatically decreasing Aβ levels in Tg6799 mice, also known 5xFAD mice, which have five familial AD (FAD) mutations. Remarkably, these effects correlated with reversal of disease-related microglial neuroinflammation, as evidenced by shifting microglia phenotypes from the inflammatory form to the anti-inflammatory form and inhibiting the nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain containing 3 inflammasome activity. Moreover, PK administration induced silent information regulator type1/peroxisome proliferator-activated receptor-γ signaling, resulting in a decrease of β-secretase 1 (BACE1) expression, which involved in Aβ production. Overall, this study suggests that PK exhibits a neuroprotective effect by inducing alternative activation of microglia and downregulating the BACE1 expression, thereby ameliorating the disease pathophysiology and reversing the cognitive decline related to Aβ deposition in AD mice.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is the leading progressive neurodegenerative disease affecting over 24 million people worldwide and is projected to affect almost 50 million people by 2030 [1]. AD is characterized by memory and cognitive dysfunction, although the underlying causes of these symptoms remain unclear [2]. Amyloid-β (Aβ) is regarded as a crucial factor in the pathogenesis of AD because Aβ plaques are found in the brain of patients with AD [3]. Reportedly, the behavioral symptoms of AD correlate with the Aβ accumulation, which trigger the inflammation-related pathway, neuronal damage, and synaptic loss [4]. To date, several studies have indicated that neuroinflammation contributes to the AD pathogenesis; thus, therapeutic strategies aimed at manipulating this inflammatory cascade, including Aβ immunization and modulation of microglial activation, which is expected decrease the AD pathology.
Currently, Food and Drug Administration-approved drugs for AD treatment are acetylcholinesterase inhibitors (donepezil, rivastigmine, and glantamine) and glutamate receptor antagonist (memantine), which offer only symptomatic treatment without disease-modifying effect [5]. However, no effective treatment exists for AD to modulate neuroinflammation and prevent cell death. Hence, further research is warranted to develop AD-modifying therapeutic agent necessary to decrease the AD pathology and enhance the cognitive function.
Picrorhiza kurroa Bentham, Scrophulariae (PK) is a famous herb in the Ayurvedic system of medicine. Several studies have demonstrated that PK exhibits therapeutic effects on several diseases through its antioxidant, anti-inflammatory, antiallergic, hepatoprotective, choleretic, antiasthmatic, and anticancerous properties [6]. Siddiqi et al. reported that PK is a good anti-inflammatory and nephroprotective alternative medicine for nimesulide toxicity [7]. In RAW 264.7 murine macrophages, PK extract suppressed nuclear factor-kappa light chain enhancer of activated B cells (NF-κB) signaling induced by lipopolysaccharide [8]. In addition, iridoid glycosides fraction from PK exerted ameliorative effects on the renal toxicity and peripheral neuropathy in cyclophosphamide-injected mice [9]. Reportedly, PK inhibited experimental arthritis by anti-inflammatory and antioxidant activity [10]. In a clinical study, PK decreases serum bilirubin and transaminases in patients with viral hepatitis [11]. Despite its various pharmacological activities, no study, to date, has assessed whether PK can affect memory impairment or neuroinflammation induced by Aβ accumulation. Hence, this study aims to scientifically assess the ability of PK on Aβ pathology and elucidate the underlying mechanism of its activity in five familial AD (5xFAD) transgenic mouse model of AD.
Materials and Methods
We purchased thioflavin S (ThS), Triton X-100, paraformaldehyde (PFA) from Sigma-Aldrich (St. Louis, MO, USA). In addition, RIPA buffer (89901) and protease and phosphatase inhibitor cocktail (1861284) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Polyvinylidene difluoride (PVDF) was purchased from Millipore (Burlington, MA, USA). A rabbit antibody against the glial fibrillary acidic protein (GFAP; Z0334) was purchased from Dako (Carpineria, CA, USA). A rabbit antibody against ionized calcium-binding adaptor molecule-1 (Iba-1; 019-19741) was purchased from Wako Chemical (Osaka, Japan). A rabbit antibody against insulin-degrading enzyme (IDE; ab32216) was purchased from Abcam (Cambridge, UK). A rabbit antibody against apoptosis-associated speck-like protein containing a carboxyterminal CARD (ASC; AG-25B-0006) was purchased from AdipoGen Life Sciences (San Diego, CA, USA). Moreover, Rabbit antibodies against silent information regulator type 1 (SIRT1; 2028s), peroxisome proliferator-activated receptor-γ (PPAR-γ; 2443s), and presenilin 1 (PS1; 5643s) were purchased from Cell Signaling Technology (Danvers, MA, USA). A mouse antibody against β-secretase1 (BACE1; MAB5308) was purchased from Millipore. A mouse antibody against amyloid precursor protein (APP; 803001) was purchased from BioLegend (San Diego, CA, USA). Mouse antibodies against nucleotide-binding oligomerization domain-like receptor family of protein 3 (NLRP3; AG-20B-0014-C100) and caspase-1 (AG-20B-0042-C100) were purchased from AdipoGen Life Sciences. Furthermore, a goat antibody against neprilysin (AF1126) was purchased from R&D Systems (Minneapolis, MN, USA), and β-actin (sc-47778HRP) and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Alexa Fluor secondary antibodies were purchased from Thermo Fisher Scientific.
Preparation and Standardization of PK
We purchased dried PK from Jungdo Herbal Drug Co. Ltd. (Gyeonggi, Republic of Korea). PK was deposited in the herbarium of the College of Pharmacy, Kyung Hee University (Seoul, Republic of Korea). Dried PK was boiled with distilled water for 2 h, filtered, evaporated in a rotary vacuum evaporator, and lyophilized to powder (yield, 13.60%). Then, the powder was stored at – 20 °C and dissolved in the appropriated vehicle before each experiment.
Animals and Treatment
We purchased 5xFAD mice from The Jackson Laboratory (Bar Harbor, ME, USA). For the experiments, we used male and female wild-type (WT) and 5xFAD mice of 5 months. All mice were housed in plastic containers under constant temperature (23 °C ± 1 °C), humidity (60 °C ± 10 °C), a 12-h light/dark cycle, and free access to food and water. 5xFAD mutations include APP KM670/671NL (Swedish), APPI716V (Florida), APPV717I (London), PSEN1 M146L, and PSEN1 L286V, resulting in early and aggressive Aβ accumulation related to neuroinflammation and memory deficits [12]. In addition, PK at 200 mg/kg/day dissolved in saline (0.9% NaCl) was orally administered using a Zonde (JD-S-124, Jeung-Do Bio & Plant Co., LTD, Seoul, Republic of Korea) for 8 weeks (Fig. 1a). In this study, all animal studies were conducted in accordance with the “Principles of Laboratory Animal Care” (NIH publication number 80-23, revised 1996) and approved by the “Animal Care and Use Guidelines” of Kyung Hee University (approval number: KHUASP(SE)-17-126-1).
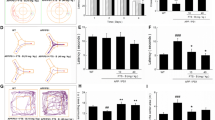
Picrorhiza kurroa Bentham, Scrophulariae (PK) ameliorates memory impairment in 5xFAD mice. (a) Protocol of the PK treatment in 5xFAD mice. (b) Escape latencies of wild-type (WT) or 5xFAD mice treated with saline or PK over 7 days (saline-treated WT mice, n = 15; PK-treated WT mice, n = 11; saline-treated 5xFAD mice, n = 15; PK-treated 5xFAD mice, n = 12). (c) Representative swimming paths on day 8 of training. (d–g) In probe trial day 8, spending time in the target quadrant (d), crossing the platform number (e), swim speed (f), and total distance (g) were recorded and analyzed. The statistical analysis included the one-way analysis of variance and Tukey’s post hoc test. #P < 0.05 versus saline-treated WT mice and *P < 0.05 versus saline-treated 5xFAD mice. Values are mean ± standard error of the mean (SEM)
Morris Water Maze Test
We performed the Morris water maze (MWM) task to evaluate the spatial memory performance. The water maze was a white tank (1.0-m diameter, 30-cm height) filled with water to a depth of 20 cm (22–24 °C). We added white opaque nontoxic paint to the water to hinder visibility. In addition, a submerged Plexiglas platform (10-cm diameter; 6–8 mm below the surface of the water) was located at a fixed position throughout the training session. Notably, the position of the platform varied from mouse to mouse while being counterbalanced across experiment groups. In this study, all mice were habituated to the maze 1 day before training, and all animals were subjected to three trials per day. A training session comprised a series of three trials per day for 7 consecutive days (total 21 trials). In each of the three trials, animals were randomly placed at different starting positions equally spaced around the perimeter of the pool. Mice were given 60 s to find the submerged platform. If mice did not mount the platform within 60 s, it was guided to the platform. The time to mount the platform was recorded as the latency for each trial. All mice were allowed to remain on the platform for 10 s before being returned to a home cage. A single probe trial, in which the platform was removed, was performed after the hidden platform task had been completed (day 8). Each mouse was placed into one quadrant of the pool and allowed to swim for 60 s. All trials were recorded using a charge-coupled device camera connected to a video monitor and a computer.
Brain Tissue Preparation
After behavioral testing, all mice were anesthetized and killed. Animals were immediately cardiac-perfused with 4% PFA in phsphate buffer. After perfusion, brains of mice were removed, postfixed overnight at 4 °C, and incubated in 30% sucrose at 4 °C until equilibrated. Next, sequential 30-μm coronal sections were cut on a freezing microtome (Leica, Wetzlar, Germany) and stored at − 20 °C.
RNA Isolation and Quantitative Real-Time PCR
mRNA transcription of cytokines was analyzed by quantitative real-time PCR (qRT-PCR). Using the Hybrid-R (GeneAll, Seoul, Republic of Korea), we extracted the total RNA from the hippocampus of mice and measured the concentration using NanoDrop ND-1000 spectrophotometer. Next, RNA samples (3 μg) were converted to cDNA using TOPscript RT DryMIX (Enzynomics, Daejeon, Republic of Korea). The cDNA was subjected to qRT-PCR using TOPreal qPCR 2× PreMIX (SYBR Green; Enzynomics) and the CFX Connect Real-Time PCR System (Bio-Rad Laboratories, Hercules, CA, USA). Supplementary Table 1 describes primers, synthesized at COSMO Genetech (Seoul, Republic of Korea).
Western Blot Analysis
The hippocampus was isolated from saline-treated WT mice, saline-treated 5xFAD mice, and PK-treated 5xFAD mice after behavioral testing. Next, the hippocampus was weighed and lysed in 10× volume of the RIPA lysis buffer plus protease and phosphatase inhibitors. Then, equal amounts of protein samples (20 μg) in the sample buffer were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred electrophoretically to immunoblotting PVDF membranes. The membranes were then pretreated with the blocking solution (5% skim milk, 0.1% Tween 20 in Tris-buffered saline, TBS) for 1 h at room temperature and reacted with primary antibodies in the blocking solution overnight at 4 °C. Next, they were washed with a washing solution (0.1% Tween 20 in TBS) five times for 10 min each and reacted with HRP-conjugated secondary antibodies against mouse, rabbit, or goat IgG in the blocking solution for 1 h at room temperature. Then, the membranes were rewashed with the washing solution five times for 10 min each. The protein signals were detected by enhanced chemiluminescence reagent (Bio-Rad Laboratories) and visualized by ChemiDoc (Bio-Rad Laboratories). After that, we detected β-actin in the same blot as an internal control for the normalization of protein loading. The intensity of bands was quantified using the ImageJ software (Bethesda, MD, USA).
ELISA Analysis
We performed Aβ1–42 enzyme-linked immunosorbent assays (ELISA) using fluorescent-based ELISA kits (Invitrogen, Carlsbad, CA, USA) and appropriate Aβ standards based on the manufacturer’s protocol. To extract insoluble faction, we homogenized the hippocampus in guanidine buffer at a final concentration of 50 mM Tris and 5 M guanidine HCl, pH 8.0. Then, homogenates were mixed at room temperature for 4 h. To obtain soluble faction, hippocampal samples were extracted in RIPA buffer. Next, homogenates were diluted in phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA), 0.03% Tween 20, and protease and phosphatase inhibitor cocktail. In this study, each Aβ standard and the experimental sample were run in duplicate, and the results were averaged.
Thioflavin S Staining
We incubated free-floating sections for 5 min at a concentration of 0.5% ThS dissolved in 50% ethanol, and then washed two times with 50% ethanol for 5 min each and once with distilled water for 5 min, and mounted with mounting medium.
Immunofluorescence
We incubated free-floating sections for 1 h in PBS containing 5% normal goat serum, 2% BSA, and 0.4% Triton X-100. In the same buffer solution, the sections were then incubated for 24 h in primary antibodies at 4 °C. For visualization, we developed the primary antibody by incubating with Alexa Fluor 488- or 594-conjugated secondary antibodies for 1 h at room temperature. Images were captured with K1-Fluo confocal microscope (Nanoscope Systems, Daejeon, Republic of Korea).
Statistical Analysis
The results from three independent experiments are summarized and presented as mean ± standard error of the mean (SEM). Comparisons between two groups were performed with Student’s t test. We performed statistical comparisons among different assays using one-way ANOVA followed by Tukey’s multiple comparison post hoc test and considered P < 0.05 as significantly different from the relative controls, as indicated by an asterisk. All statistical analyses in this study were performed using the GraphPad Prism 5.0 software (Graph Pad Software Inc., San Diego, CA, USA). No significant difference between male and female mice in all analyses; thus, data obtained from male and female mice were combined.
Results
PK Treatment Enhances Cognitive Impairments in 5xFAD Mice
To examine whether PK influences memory dysfunction in 5xFAD mice, saline- or PK-treated 5xFAD mice and their WT littermates were tested using MWM task, which is a widely used method to measure hippocampus-dependent learning and memory of mice [13]. Figure 1a summarizes the protocol. We assessed spatial memory by determining the escape time in the hidden platform of three trials per day. In this study, 5xFAD mice exhibited markedly impaired learning and memory compared with WT mice. However, we determined that PK-administered 5xFAD mice exhibited markedly faster escape times than saline-treated 5xFAD mice on day 7 (Fig. 1b, c). In addition, representative navigation paths on day 8 of training provided evidence that spatial learning acquisition was impaired in 5xFAD mice that received saline compared with PK-treated 5xFAD mice, which exhibited a navigation pattern similar to WT mice (Fig. 1c). On day 8, we evaluated the number of crossing platform, and time spent in the target quadrant during 60 s to assess whether mice used a nonspatial strategy to find the platform. We observed that the PK administration enhanced memory deficits without decreasing the locomotor activity (Fig. 1d–g).
PK Treatment Decreases Aβ Burden in the Hippocampus of 5xFAD Mice
We analyzed the Aβ deposition using ThS staining and 6E10 immunostaining in the cortex and hippocampus to investigate whether memory improvements by the PK administration correlated with the Aβ pathology. We observed a marked reduction in the area occupied by the aggregated Aβ in the hippocampus of PK-treated 5xFAD mice compared with saline-treated counterparts, and no significant changes were noted in the cortex (Fig. 2a–d). In addition, the ELISA analysis was performed to confirm that the staining results were an accurate reflection of the quantitative change in brain Aβ protein levels. The levels of Aβ 40 and 42 are elevated early in dementia, and this change has been markedly correlated with a cognitive decline. Some studies have reported Aβ 42 to be the most toxic Aβ isoform that elicits an immunological response [14] and cognitive deficit [15]. In the present study, the ELISA results demonstrated that the brain of PK-treated 5xFAD mice had reduced significantly both soluble and insoluble Aβ 42 levels compared with those of saline-infused 5xFAD mice (Fig. 2e). Our data provide an unambiguous evidence that PK prevents the formation of Aβ or clears the Aβ deposits in the brain of the AD mice model.
Picrorhiza kurroa Bentham, Scrophulariae (PK) leads to reduce Aβ pathology in the hippocampus of 5xFAD mice. Brain sections from 7-month-old mice were stained with thioflavin S (ThS) and anti-6E10 antibody. (a) Representative images of ThS staining in the cortex and hippocampus; scale bar, 100 μm. (b) The quantification of ThS-stained area (n = 5–6 per group). (c) Immunofluorescence images of 6E10 staining in the cortex and hippocampus; scale bar, 100 μm. (d) The quantification of the 6E10-positive area (n = 5–6 per group). (e) Analysis of soluble and insoluble Aβ1–42 levels from the mice hippocampus using ELISA kits (n = 6 per group). The statistical analysis included the Student’s t test. *P < 0.05 and **P < 0.01 versus saline-treated 5xFAD mice. Values are mean ± standard error of the mean (SEM)
PK Treatment Inhibits Microglia Activation in the Hippocampus
Reportedly, neuroinflammation could contribute to the AD pathology by promoting the Aβ generation and accumulation [16]. To date, several studies have reported that PK could decrease inflammatory responses [8, 10]. Thus, we assessed whether PK could regulate neuroinflammation in AD mice. Accordingly, we performed immunostaining using GFAP and Iba-1 antibodies to investigate the change in neuroinflammation. PK did not affect GFAP expression levels, suggesting that PK could not regulate the astrocyte activation (Fig. 3a–d). However, Iba-1-positive cells were markedly decreased in the hippocampus of PK-treated AD mice than saline-infused mice (Fig. 3e, f). We observed similar results in the western blot analysis (Fig. 3g, h).
Picrorhiza kurroa Bentham, Scrophulariae (PK) inhibits microglia activation, but not the astrocyte activation in the hippocampus of 5xFAD mice. Brain sections from 7-month-old mice were stained with anti-GFAP (anti-glial fibrillary acidic protein) and Iba-1 antibody. Immunofluorescence images of GFAP (a) and Iba-1 (e) staining in the hippocampus; scale bar, 100 μm. (b, f) The quantification of GFAP-positive- and Iba-1-positive cell area (n = 4–6 per group). Mouse brain lysates were analyzed for GFAP (c) and Iba-1 (g) levels using the western blot analysis. The quantification of GFAP (d) and Iba-1 (h) levels (n = 5–6 per group). The statistical analysis included the one-way analysis of variance and Tukey’s post hoc test. ###P < 0.001 versus saline-treated wild-type (WT) mice and *P < 0.05 versus saline-treated 5xFAD mice. Values are mean ± standard error of the mean (SEM)
Microglia exhibit different phenotypes, either pro-inflammatory or anti-inflammatory, based on the microenvironment [17, 18]. We examined pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) and anti-inflammatory cytokines (IL-4, IL-10, and FIZZ1) to investigate whether PK affects the pro-/anti-inflammatory phenotypes in AD mice brain. As anticipated, 5xFAD mice exhibited elevated pro-inflammatory cytokines levels compared with that of WT mice. Contrarily, PK-treated mice exhibited marginally reduced TNF-α and IL-6 levels (Fig. 4a, b) and markedly reduced IL-1β mRNA expression and protein levels (Fig. 4c, d). In addition, anti-inflammatory cytokines, such as IL-4, IL-10, and FIZZ1, increased by PK treatment in 5xFAD mice (Fig. 4e–g). Overall, these findings suggested that a decline of Aβ accumulation after PK treatment could correlate with decreasing neuroinflammation by modulating microglia phenotypes.
Picrorhiza kurroa Bentham, Scrophulariae (PK) modulates microglia phenotypes in the hippocampus of 5xFAD mice. The inflammatory (a–c) and anti-inflammatory (e–g) cytokines were measured in the hippocampus with quantitative qRT-PCR (n = 5–6 per group). (d) The protein level of IL-1β was analyzed in hippocampus lysate by the ELISA kit (n = 5–6 per group). The statistical analysis included the one-way analysis of variance and Tukey’s post hoc test. ###P < 0.001 versus saline-treated wild-type (WT) mice and *P < 0.05 and **P < 0.01 versus saline-treated 5xFAD mice. Values are mean ± standard error of the mean (SEM)
PK Treatment Inhibits Inflammasome Signaling in the Hippocampus
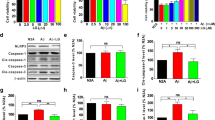
In this study, we observed that PK could decrease the mRNA and protein expression levels of IL-1β. The maturity and secretion of IL-1β depend on the activation of NLRP3 inflammasomes [19]. Thus, one possible mechanism underlying pro-inflammatory cytokine inhibition is the suppression of the activation of NLRP3 inflammasomes by PK. We first performed the western blot analysis to examine the activation of NLRP3 inflammasomes in 5xFAD mice; the results revealed that the protein expression of NLRP3 in 5xFAD mice was markedly increased compared with WT mice, but this protein was markedly decreased by PK treatment. In addition, the cleaved caspase-1 level was upregulated in 5xFAD mice compared with WT mice, but this was decreased in PK-treated 5xFAD mice (Fig. 5a–d). Furthermore, ASC expression levels did not differ between groups (Fig. 5c). These findings suggest that PK blocked the release of IL-1β, a significant factor of neuroinflammation by inhibiting the NLRP3 signaling platform activity.
Picrorhiza kurroa Bentham, Scrophulariae (PK) inhibits the NLRP3 inflammasome pathway in the hippocampus of 5xFAD mice. (a) The western blot analysis of NLRP3, ASC, and caspase-1 (Casp1) levels in the hippocampus of 7-month-old mice. (b–d) The quantification of NLRP3 (b), ASC (c), pro-Casp1, and cleaved Casp1 (d) were normalized to β-actin (n = 5–6 per group). The statistical analysis included the one-way analysis of variance and Tukey’s post hoc test. #P < 0.05 and ##P < 0.01 versus saline-treated wild-type (WT) mice and *P < 0.05 and **P < 0.01 versus saline-treated 5xFAD mice. Values are mean ± standard error of the mean (SEM)
PK Treatment Reduces the BACE1 Expression by the SIRT1–PPAR-γ Pathway
A reduction of Aβ deposits in 5xFAD mice after PK treatment could be attributed to an elevation of Aβ-degrading enzymes (IDE and neprilysin) released by microglia. Accordingly, we analyzed protein and mRNA levels of Aβ-degrading enzymes to examine whether PK-induced phenotypic changes of microglia affect Aβ clearance; however, we did not detect differences between groups (Fig. 6). Another possible cause of Aβ reduction by PK could be associated with generating a process of Aβ. We analyzed protein expression of APP, PS1, and BACE1 to address whether PK affects Aβ processing. We observed that the PK administration decreased BACE1 mRNA and protein levels without changes in the expression levels of APP and PS1 (Fig. 7). Reportedly, the SIRT1–PPAR-γ pathway modulates the BACE1 expression [20]. Hence, we assessed protein levels of these factors by the western blot analysis. Furthermore, PK-treated 5xFAD mice exhibited markedly elevated expression of SIRT1 and PPAR-γ compared with saline-treated 5xFAD mice (Fig. 8). These findings suggest that the inhibitory effect of Aβ deposits after PK treatment might be mediated by the downregulation of BACE1 through the induction of the SIRT1–PPAR-γ pathway.
Picrorhiza kurroa Bentham, Scrophulariae (PK) do not affect Aβ-degrading enzymes expression in the hippocampus of 5xFAD mice. (a, b) The protein expression of insulin-degrading enzyme (IDE) (a) and neprilysin (b) were determined by the western blot analysis (n = 5–6 per group). (c, d) The mRNA expression of IDE (c) and neprilysin (d) were measured by qRT-PCR (n = 5–6 per group). The statistical analysis included the one-way analysis of variance and Tukey’s post hoc test. #P < 0.05 and ###P < 0.001 versus saline-treated wild-type (WT) mice. Values are mean ± standard error of the mean (SEM)
Picrorhiza kurroa Bentham, Scrophulariae (PK) inhibits amyloid precursor protein (APP) processing in the hippocampus of 5xFAD mice. (a) The western blot analysis of APP, C99, and presenillin1 levels in the hippocampus of 7-month-old mice. (b–d) The quantification of APP (d), C99 (c), and presenillin1 (d) were normalized to β-actin (n = 5–6 per group). (e) The mRNA of BACE1 was measured by qRT-PCR. (f) The western blot analysis of Pro-BACE1 and BACE1 levels in the hippocampus of 7-month-old mice. (g, h) The quantification of Pro-BACE1 (g) and BACE1 (h) were normalized to β-actin (n = 5–6 per group). The statistical analysis included the one-way analysis of variance and Tukey’s post hoc test. #P < 0.05, ##P < 0.01, and ###P < 0.001 versus saline-treated wild-type (WT) mice and *P < 0.05 and **P < 0.01 versus saline-treated 5xFAD mice. Values are mean ± standard error of the mean (SEM)
Discussion
Excessive neuroinflammation in AD is triggered by emerging senile plaques and tau tangles and accelerate to more pathogenesis of Aβ and tau protein themselves [21]. Human AD brain samples from early stages expressed inflammatory cytokines, including TNF-α, IL-6, and IL-1β [22]. Thus, decreasing neuroinflammation is one of the therapeutic strategies to delay or relieve AD neuropathology [23]. Several studies have suggested the beneficial properties of PK in preventing inflammation-mediated diseases [10, 24, 25]. In addition, PK extracts exert an anti-inflammatory effect through the suppression of NF-κB signaling in lipopolysaccharide-stimulated RAW 264.7 murine macrophages [8]. In cyclophosphamide-injected mice, iridoid glycosides fraction of PK attenuates the renal toxicity and peripheral neuropathy by modulating PPAR-γ-mediated inflammatory signaling [9]. However, the impact of PK on AD and related mechanisms remain unknown. To the best of our knowledge, this is the first study to demonstrate that PK ameliorates Aβ-associated pathology through anti-inflammatory effects in 5xFAD mice.
Activated microglia induced by Aβ can release dangerous factors, such as TNF-α, IL-6, and IL-1β, resulting in pathological processes, which is a common pathological characteristic of AD patients and animal models [26, 27]. Based on the activated phenotypes, microglia can release cytotoxic cytokines or neurotrophic cytokines. In particular, Aβ-activated microglia finally lead to neuronal loss [28]. Zhang and He reported that modulating of microglia phenotypes attenuates Aβ deposits and memory impairments in APP/PS1 mice [29]. We observed that PK-administrated 5xFAD mice had decreased pro-inflammatory factor, IL-1β, and elevated anti-inflammatory cytokines, including FIZZ1, IL-4, and IL-10. These findings suggest that PK restored microglia homeostasis by regulating the homeostasis of two phenotypes of microglia.
The NLRP3 inflammasome, a multiprotein complex that includes NLRP3-recognizing harmful signals, adaptor protein ASC, and pro-caspase-1 known to activate the protein of inflammasome, is a crucial signaling mediator in the inflammatory activation [19]. NLRP3 is highly expressed in microglia and is implicated in multiple chronic inflammatory diseases as it can sense inflammatory crystals and aggregated proteins, including Aβ [30]. A study recently reported that genetic deficiency of NLRP3 inflammasome-related factors ameliorates memory and behavior deficits in APP/PS1 mice. In addition, NLRP3 and caspase-1 gene deficiency results in declined Aβ levels. Reportedly, the protein levels of cleaved caspase-1 are elevated in the hippocampus and cortex of patients with AD compared with controls [27]. In particular, mature IL-1β form is generated by the inflammasome, and a higher concentration has been detected in the cerebrospinal fluid of patients with AD [31,32,33]. Thus, several studies have investigated the inhibition of the NLRP3 inflammasome for AD therapy. For example, Dempsey et al. reported that the inhibition of the NLRP3 inflammasome using small molecule enhances the clearance of Aβ and memory function [34]. In a study, virgin coconut oil exhibited positive effects on Aβ- and/or high fat-induced learning and memory dysfunction by reducing mRNA levels of NLRP3 inflammasome-related factors [35]. Furthermore, pterostilbene decreased the inflammatory cytokine production by inhibiting NLRP3/caspase-1 inflammasome induced by Aβ1–42 in BV2 microglial cells [36]. This study found that the protein expressions of NLRP3 and caspase-1 were markedly increased in 5xFAD mice. The PK treatment effectively reduced the levels of IL-1β, which was attributed to the downregulation of NLRP3 protein and inhibition of caspase-1 activity. These findings, as mentioned above, indicated that the decrease of the microglia activation by PK might be associated with its inhibition of the NLRP3 inflammasome activation. However, the exact signaling mechanisms resulting in inhibitory effects of the NLRP3 inflammasome by PK warrant further elucidation.
The inhibition of neuroinflammation modulates APP processing and Aβ clearance [37]. We analyzed Aβ protein levels and deposition in the hippocampus and cortex of 5xFAD mice to investigate the effects of PK on Aβ pathological changes. The PK administration for 2 months decreased the protein levels and plaque areas of Aβ in the hippocampus, but not in the cortex. We performed qRT-PCR and western blot analysis to determine whether PK affected APP processing factors and Aβ-degrading enzymes expression. Protein and mRNA levels of IDE and neprilysin contributing to the Aβ degradation were not changed in PK-treated 5xFAD mice compared with saline-treated counterparts. In addition, amyloidogenic processing of APP involves sequential cleavages by BACE1 and γ-secretase at the N and C termini of Aβ, respectively. The 99-amino-acid C-terminal fragment of APP generated by the BACE1 cleavage can be internalized and further processed by γ-secretase to produce Aβ 40/42. To date, several studies have indicated that the neuroinflammatory reaction could contribute to the BACE1 expression [16, 38]. Remarkably, this study demonstrates that the PK administration downregulated the protein level of the BACE1 protein expression in 5xFAD mice through the neuroinflammatory modulation and that the decreased BACE1 expression could diminish the Aβ formation. We further analyzed the SIRT1–PPAR-γ signaling process, known to regulate BACE1, to elucidate the mechanisms underlying the effects of PK on the BACE1 expression [20]. Reportedly, PPAR-γ agonists affect Aβ levels by modulating the BACE1 expression in the AD animal model [39]. Marwarha et al. reported that leptin inhibits the BACE1 expression by the activation of SIRT1 signaling in neuronal cells [40]. Similar to a previous study, PK could induce the SIRT1–PPAR-γ pathway. Overall, these findings suggest that PK can reduce Aβ levels through the regulation of the BACE1 expression by the activation of the SIRT1–PPAR-γ signaling pathway.
In conclusion, this study suggests that PK could rescue cognitive impairment, decrease excessive neuroinflammation, regulate microglia phenotype, and alleviate Aβ levels in 5xFAD mice. In addition, the mechanisms underlying the therapeutic effects of PK in AD might involve the inhibition of NLRP3 inflammasome-mediated microglia activation and reduction of the BACE1 expression. The twin effects of PK in inhibiting APP processing and NLRP3 inflammasome signals result in alleviating cognitive deficits. Overall, this study provides strong evidence that PK could effectively interfere with the AD progression.
References
Winblad, B., Amouyel, P., Andrieu, S., Ballard, C., Brayne, C., Brodaty, H., et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016,15:455-532.
Donev, R., Kolev, M., Millet, B., Thome, J. Neuronal death in Alzheimer’s disease and therapeutic opportunities. J Cell Mol Med. 2009,13:4329-48.
Sevigny, J., Chiao, P., Bussiere, T., Weinreb, P. H., Williams, L., Maier, M., et al. Addendum: The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2017,546:564.
Bloom, G. S. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014,71:505-8.
Casey, D. A., Antimisiaris, D., O’Brien, J. Drugs for Alzheimer’s disease: are they effective? P T. 2010,35:208-11.
Picrorhiza kurroa. Monograph. Altern Med Rev. 2001,6:319-21.
Siddiqi, A., Nazneen, Z., Haqnawaz, Alam, S. S., Tariq, S. Mechanism of nephroprotection By Picrorhiza kurroa. J Ayub Med Coll Abbottabad. 2018,30:184-6.
Kumar, R., Gupta, Y. K., Singh, S., Raj, A. Anti-inflammatory effect of Picrorhiza kurroa in experimental models of inflammation. Planta Med. 2016,82:1403-9.
Sharma, S., Sharma, P., Kulurkar, P., Singh, D., Kumar, D., Patial, V. Iridoid glycosides fraction from Picrorhiza kurroa attenuates cyclophosphamide-induced renal toxicity and peripheral neuropathy via PPAR-gamma mediated inhibition of inflammation and apoptosis. Phytomedicine. 2017,36:108-17.
Kumar, R., Gupta, Y. K., Singh, S., Arunraja, S. Picrorhiza kurroa inhibits experimental arthritis through inhibition of pro-inflammatory cytokines, angiogenesis and MMPs. Phytother Res. 2016,30:112-9.
Vaidya, A. B., Antarkar, D. S., Doshi, J. C., Bhatt, A. D., Ramesh, V., Vora, P. V., et al. Picrorhiza kurroa (Kutaki) Royle ex Benth as a hepatoprotective agent—experimental & clinical studies. J Postgrad Med. 1996,42:105-8.
Oakley, H., Cole, S. L., Logan, S., Maus, E., Shao, P., Craft, J., et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006,26:10129-40.
Vorhees, C. V., Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006,1:848-58.
Simard, A. R., Soulet, D., Gowing, G., Julien, J. P., Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006,49:489-502.
Koistinaho, M., Ort, M., Cimadevilla, J. M., Vondrous, R., Cordell, B., Koistinaho, J., et al. Specific spatial learning deficits become severe with age in beta-amyloid precursor protein transgenic mice that harbor diffuse beta-amyloid deposits but do not form plaques. Proc Natl Acad Sci U S A. 2001,98:14675-80.
Lee, J. W., Lee, Y. K., Yuk, D. Y., Choi, D. Y., Ban, S. B., Oh, K. W., et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008,5:37.
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J., Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000,164:6166-73.
Cherry, J. D., Olschowka, J. A., O’Banion, M. K. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014,11:98.
Schroder, K., Tschopp, J. The inflammasomes. Cell. 2010,140:821-32.
Wang, R., Li, J. J., Diao, S., Kwak, Y. D., Liu, L., Zhi, L., et al. Metabolic stress modulates Alzheimer’s beta-secretase gene transcription via SIRT1-PPARgamma-PGC-1 in neurons. Cell Metab. 2013,17:685-94.
Zhang, B., Gaiteri, C., Bodea, L. G., Wang, Z., McElwee, J., Podtelezhnikov, A. A., et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013,153:707-20.
Sudduth, T. L., Schmitt, F. A., Nelson, P. T., Wilcock, D. M. Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiol Aging. 2013,34:1051-9.
Szekely, C. A., Zandi, P. P. Non-steroidal anti-inflammatory drugs and Alzheimer’s disease: the epidemiological evidence. CNS Neurol Disord Drug Targets. 2010,9:132-9.
Kantibiswas, T., Marjit, B., Maity, L. N. Effect of Picrorhiza kurroa Benth. In acute inflammation. Anc Sci Life. 1996,16:11-4.
Pandey, B. L., Das, P. K. Immunopharmacological studies on picrorhiza kurroa Royle-Ex-Benth. Part V: anti-inflammatory action: relation with cell types involved in inflammation. Indian J Physiol Pharmacol. 1988,32:289-92.
Pawate, S., Shen, Q., Fan, F., Bhat, N. R. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004,77:540-51.
Heneka, M. T., Kummer, M. P., Stutz, A., Delekate, A., Schwartz, S., Vieira-Saecker, A., et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013,493:674-8.
Tang, Y., Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016,53:1181-94.
Zhang, Y., He, M. L. Deferoxamine enhances alternative activation of microglia and inhibits amyloid beta deposits in APP/PS1 mice. Brain Res. 2017,1677:86-92.
Song, L., Pei, L., Yao, S., Wu, Y., Shang, Y. NLRP3 inflammasome in neurological diseases, from functions to therapies. Front Cell Neurosci. 2017,11:63.
Akama, K. T., Van Eldik, L. J. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000,275:7918-24.
Blum-Degen, D., Muller, T., Kuhn, W., Gerlach, M., Przuntek, H., Riederer, P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett. 1995,202:17-20.
Rubio-Perez, J. M., Morillas-Ruiz, J. M. A review: inflammatory process in Alzheimer’s disease, role of cytokines. Scientific World Journal. 2012,2012:756357.
Dempsey, C., Rubio Araiz, A., Bryson, K. J., Finucane, O., Larkin, C., Mills, E. L., et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun. 2017,61:306-16.
Mirzaei, F., Khazaei, M., Komaki, A., Amiri, I., Jalili, C. Virgin coconut oil (VCO) by normalizing NLRP3 inflammasome showed potential neuroprotective effects in amyloid-beta induced toxicity and high-fat diet fed rat. Food Chem Toxicol. 2018,118:68-83.
Li, Q., Chen, L., Liu, X., Li, X., Cao, Y., Bai, Y., et al. Pterostilbene inhibits amyloid-beta-induced neuroinflammation in a microglia cell line by inactivating the NLRP3/caspase-1 inflammasome pathway. J Cell Biochem. 2018,119:7053-62.
Kummer, M. P., Vogl, T., Axt, D., Griep, A., Vieira-Saecker, A., Jessen, F., et al. Mrp14 deficiency ameliorates amyloid beta burden by increasing microglial phagocytosis and modulation of amyloid precursor protein processing. J Neurosci. 2012,32:17824-9.
He, P., Zhong, Z., Lindholm, K., Berning, L., Lee, W., Lemere, C., et al. Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer’s mice. J Cell Biol. 2007,178:829-41.
Sastre, M., Dewachter, I., Rossner, S., Bogdanovic, N., Rosen, E., Borghgraef, P., et al. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci U S A. 2006,103:443-8.
Marwarha, G., Raza, S., Meiers, C., Ghribi, O. Leptin attenuates BACE1 expression and amyloid-beta genesis via the activation of SIRT1 signaling pathway. Biochim Biophys Acta. 2014,1842:1587-95.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1B07050547), the Medical Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (NRF-2017R1A5A2014768), and the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through High Value-Added Food Technology Development Program, funded by Ministry of Agriculture Food and Rural Affairs (MAFRA) (318027-04).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Contributions
N.K.K. and J.M.D. performed the experiments, analyzed the data, and prepared the manuscript. I.G.J. and S.H.J. performed the animal study. M.S.O. and J.K.L. interpreted the data and reviewed the manuscript. M.S.O. designed the study and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, N., Do, J., Ju, I.G. et al. Picrorhiza kurroa Prevents Memory Deficits by Inhibiting NLRP3 Inflammasome Activation and BACE1 Expression in 5xFAD Mice. Neurotherapeutics 17, 189–199 (2020). https://doi.org/10.1007/s13311-019-00792-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-019-00792-7