Abstract
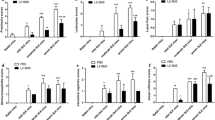
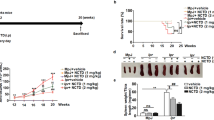
Systemic lupus erythematosus (SLE) is a chronic multi-system inflammatory disease associated with autoantibody formation. Clinical management of lupus is associated with multiple adverse events. Resveratrol is a phytoalexin with several pharmacological properties. This study aimed to evaluate the combinatorial effect of resveratrol (25 mg/kg and 50 mg/kg) and its bio-enhancer piperine (1/10th dose of resveratrol) on pristane-induced SLE murine model. Mice were injected with 0.5 ml of pristane and after 2 months they were orally dosed with resveratrol combinations for 4 months. Determined by indirect immunofluorescence, resveratrol was unable to abrogate autoantibody formation. The increased IFN-α, IL-6 and TNF-α was mitigated by low dose of resveratrol and piperine (RP-1). None of the doses regulated the increase in nitric oxide. Lipogranulomas associated with injected pristane were not observed after RP-1 and high dose of resveratrol (Res-2) treatment. Lupus mice witnessed IgG and IgM immune complexes by direct immunofluorescence assay and associated histopathological observations in kidneys, liver, lung, spleen and skin. None of the treatment regimens were able to regulate the manifestations observed in spleen and skin. RP-1 and Res-2 proved beneficial in kidney, liver and lungs and were able to ameliorate lupus associated manifestations. Renal manifestations (proteinuria and decreased creatinine in urine) were successfully mitigated by RP-1 and Res-2 and high dose combination of resveratrol and piperine. Oxidative stress (reactive oxygen species by flowcytometry and catalase, superoxide dismutase, glutathione peroxidase, reduced glutathione and lipid peroxidation by biochemical analysis) was evident by pristane injection. These were regulated by different doses of resveratrol alone and in combination with piperine. Hence, resveratrol when used in combination with piperine successfully reduces some measures of morbidity with little or no effect on mortality associated with lupus.















Similar content being viewed by others
References
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Boily G, He XH, Pearce B, Jardine K, McBurney MW (2009) SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene 28(32):2882–2893
Chowdhary VR, Grande JP, Luthra HS, David CS (2007) Characterization of haemorrhagic pulmonary capillaritis: another manifestation of Pristane-induced lupus. Rheumatology 46(9):1405–1410
Eruslanov E, Kusmartsev S (2010) Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 594:57–72
Faghihzadeh F, Hekmatdoost A, Adibi P (2015) Resveratrol and liver: A systematic review. J Res Med Sci 20(8):797–810
Fischer AH, Jacobson KA, Rose J, Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. https://doi.org/10.1101/pdb.prot4986
Grisham MB, Johnson GG, Lancaster JR Jr (1996) Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol 268:237–246
Helyer BJ, Howie JB (1963) Renal Disease associated with Positive Lupus Erythematosus Tests in a Crossbred Strain of Mice. Nature 197:197. https://doi.org/10.1038/197197a0
Jhou J-P, Chen S-J, Huang H-Y, Lin W-W, Huang D-Y, Tzeng S-J (2017) Upregulation of FcγRIIB by resveratrol via NF-κB activation reduces B-cell numbers and ameliorates lupus. Exp Mol Med 49:e381. https://doi.org/10.1038/emm.2017.144
Kalunian KC (2016) Interferon-targeted therapy in systemic lupus erythematosus: is this an alternative to targeting B and T cells? Lupus 25(10):1097–1101
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186(1):189–195
Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS (2004) Piperine enhances the bioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J Nutr 134(8):1948–1952
Luck H (1954) Quantitative determination of catalase activity of biological material. Enzymologia 17(1):31–40
Minhas U, Das P, Bhatnagar A (2011) Role of reactive intermediates in the immunopathogenesis of the pristane-induced Balb/c model of lupus. Lupus 20(13):1421–1425
Muraguchi A, Hirano T, Tang B, Matsuda T, Horii Y, Nakajima K, Kishimoto T (1988) The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med 167(2):332–344
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Ouyang Q, Huang Z, Wang Z, Chen X, Ni J, Lin L (2014) Effects of pristane alone or combined with chloroquine on macrophage activation, oxidative stress, and TH1/TH2 skewness. J Immunol Res 613136(10):6
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Pannu N, Bhatnagar A (2019) Resveratrol: from enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed Pharmacother 109:2237–2251. https://doi.org/10.1016/j.biopha.2018.11.075
Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L (2009) Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol 30(9):455–464
Satoh M, Reeves WH (1994) Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med 180(6):2341–2346
Satoh M, Kumar A, Kanwar YS, Reeves WH (1995) Anti-nuclear antibody production and immune-complex glomerulonephritis in BALB/c mice treated with pristane. Proc Natl Acad Sci U S A 92(24):10934–10938
Shah D, Mahajan N, Sah S, Nath SK, Paudyal B (2014) Oxidative stress and its biomarkers in systemic lupus erythematosus. J Biomed Sci 21(1):23
Sharma P, Huq AU, Singh R (2014) Cypermethrin-induced reproductive toxicity in the rat is prevented by resveratrol. J Hum Reprod Sci 7(2):99–106
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64(4):353–356
Shortman K, Williams N, Adams P (1972) The separation of different cell classes from lymphoid organs. V. Simple procedures for the removal of cell debris. Damaged cells and erythroid cells from lymphoid cell suspensions. J Immunol Methods 1(3):273–287
Silva AM, Oliveira MI, Sette L, Almeida CR, Oliveira MJ, Barbosa MA, Santos SG (2014) Resveratrol as a natural anti-tumor necrosis factor-α molecule: implications to dendritic cells and their crosstalk with mesenchymal stromal cells. PLoS ONE 9(3):e91406
Toora BD, Rajagopal G (2002) Measurement of creatinine by Jaffe’s reaction–determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian J Exp Biol 40(3):352–354
van Ginkel PR, Darjatmoko SR, Sareen D, Subramanian L, Bhattacharya S, Lindstrom MJ, Albert DM, Polans AS (2008) Resveratrol inhibits uveal melanoma tumor growth via early mitochondrial dysfunction. Invest Ophthalmol Vis Sci 49(4):1299–1306
Wang ZL, Luo XF, Li MT, Xu D, Zhou S, Chen HZ, Gao N, Chen Z, Zhang LL, Zeng XF (2014) Resveratrol possesses protective effects in a pristane-induced lupus mouse model. PLoS ONE 9(12):e114792
Wu CT, Yu HP, Chung CY, Lau YT, Liao SK (2008) Attenuation of lung inflammation and pro-inflammatory cytokine production by resveratrol following trauma-hemorrhage. Chin J Physiol 51(6):363–368
Zhong M, Cheng GF, Wang WJ, Guo Y, Zhu XY, Zhang JT (1999) Inhibitory effect of resveratrol on interleukin 6 release by stimulated peritoneal macrophages of mice. Phytomedicine 6(2):79–84
Funding
Funding was provided by University Grants Commission (Grant No. F4-1/2006(BSR)/7-209/2009(BSR)) and Department of Science and Technology, Ministry of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pannu, N., Bhatnagar, A. Combinatorial therapeutic effect of resveratrol and piperine on murine model of systemic lupus erythematosus. Inflammopharmacol 28, 401–424 (2020). https://doi.org/10.1007/s10787-019-00662-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-019-00662-w