Abstract
Introduction
Over the last decade, atomic force microscopy (AFM) has played an important role in understanding nanomechanical properties of various cancer cell lines. This study is focused on Lewis lung carcinoma cell tumours as 3D multicellular spheroid (MS). Not much is know about the mechanical properties of the cells and the surrounding extracellular matrix (ECM) in rapidly growing tumours.
Methods
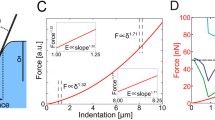
Depth-dependent indentation measurements were conducted with the AFM. Force-vs.-indentation curves were used to create stiffness profiles as a function of depth. Here studies were focused on the outer most layer, i.e., proliferation zone of the spheroid.
Results
Both surface and sub-surface stiffness profiles of MS were created. This study revealed three nanomechanical topographies, Type A-high modulus due to collagen fibers, Type B-high stiffness at cell membrane and ECM interface and Type C-increased modulus due to cell lying deep inside matrix at a depth of 1.35 μm. Both Type and Type-B topographies result from collagen-based structures in ECM.
Conclusion
This study has first time revealed mechanical constitution of an MS. Depth-dependent indentation studies have the revealed role of various molecular and cellular components responsible for providing mechanical stability to MS. Nanomechanical heterogeneities revealed in this investigation can shed new light in developing correct dosage regime for collagenase treatment of tumours and designing better controlled artificial extracellular matrix systems for replicating tissue growth in-vitro.






Similar content being viewed by others
References
Andre, F., N. Berrada, and C. Desmedt. Implication of tumor microenvironment in the resistance to chemotherapy in breast cancer patients. Curr. Opin. Oncol. LWW 22:547–551, 2010.
Bagnoli, K. L. Influence of EGF on HaCaT cells as measured by AFM with a comparison of AFM and 3D optical deconvolution using MH-S cells. M.S. Thesis, University of Connecticut, 2007.
Bancelin, S., et al. Determination of collagen fibril size via absolute measurements of second-harmonic generation signals. Nat. Commun. 5:4920, 2014.
Bertram, J. S., and P. Janik. Establishment of a cloned line of Lewis lung carcinoma cells adapted to cell culture. Cancer Lett. 11:63–73, 2017. https://doi.org/10.1016/0304-3835(80)90130-5.
Carlsson, J., and J. M. Yuhas. Liquid-overlay culture of cellular spheroids. Recent Results Cancer Res. 95:1–23, 1984.
Chaudhuri, O., et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 13:970–978, 2014. https://doi.org/10.1038/nmat4009.
Cross, S. E., Y.-S. Jin, J. Rao, and J. K. Gimzewski. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2:780–783, 2007. https://doi.org/10.1038/nnano.2007.388.
Dokukin, M. E., and I. Sokolov. On the Measurements of Rigidity Modulus of Soft Materials in Nanoindentation Experiments at Small Depth. Macromolecules 45:4277–4288, 2012. https://doi.org/10.1021/ma202600b.
Dufrene, Y. F., D. Martinez-Martin, I. Medalsy, D. Alsteens, and D. J. Muller. Multiparametric imaging of biological systems by force-distance curve-based AFM. Nat. Meth. 10:847–854, 2013. https://doi.org/10.1038/nmeth.2602.
Efremov, Y. M., A. A. Dokrunova, D. V. Bagrov, K. S. Kudryashova, O. S. Sokolova, and K. V. Shaitan. The effects of confluency on cell mechanical properties. J. Biomech. 46:1081–1087, 2013.
Gelse, K., E. Pöschl, and T. Aigner. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 55:1531–1546, 2003.
Glimelius, B., B. Norling, T. Nederman, and J. Carlsson. Extracellular matrices in multicellular spheroids of human glioma origin: increased incorporation of proteoglycans and fibronectin as compared to monolayer cultures. APMIS 96:433–444, 1988.
Goodman, T. T., P. L. Olive, and S. H. Pun. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int. J. Nanomed. 2:265–274, 2007.
Guz, N., M. Dokukin, V. Kalaparthi, and I. Sokolov. If cell mechanics can be described by elastic modulus: study of different models and probes used in indentation experiments. Biophys. J. 107:564–575, 2014.
Heim, A. J., W. G. Matthews, and T. J. Koob. Determination of the elastic modulus of native collagen fibrils via radial indentation. Appl. Phys. Lett. 89:181902, 2006.
Hoh, J. H., and C. A. Schoenenberger. Surface morphology and mechanical properties of MDCK monolayers by atomic force microscopy. J. Cell Sci. 107:1105–1114, 1994.
Jandt, K. D., M. Finke, and P. Cacciafesta. Aspects of the physical chemistry of polymers, biomaterials and mineralised tissues investigated with atomic force microscopy (AFM). Colloids Surf. B Biointerfaces 19:301–314, 2000.
Jang, S. H., M. G. Wientjes, D. Lu, and J. L.-S. Au. Drug delivery and transport to solid tumors. Pharm. Res. 20:1337–1350, 2003. https://doi.org/10.1023/A:1025785505977.
Kalluri, R. Angiogenesis: basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 3:422–433, 2003.
Keaton, A., J. F. Holzrichter, R. Balhorn, and W. J. Siekhaus. Nanostethoscopy: a new mode of operation of the atomic force microscope BT. In: Forces in Scanning Probe Methods, edited by H. J. Güntherodt, D. Anselmetti, and E. Meyer. Dordrecht: Springer, 1995, pp. 91–97. https://doi.org/10.1007/978-94-011-0049-6_8.
Kessel, S., et al. Thermoresponsive PEG-based polymer layers: surface characterization with AFM force measurements. Langmuir 26:3462–3467, 2010. https://doi.org/10.1021/la903007v.
Kim, S.-H., H.-J. Kuh, and C. R. Dass. The reciprocal interaction: chemotherapy and tumor microenvironment. Curr. Drug Discov. Technol. 8:102–106, 2011.
Kühn, K., et al. Macromolecular structure of basement membrane collagens. FEBS Lett. 125:123–128, 1981.
Kwon, E.-Y., Y.-T. Kim, and D.-E. Kim. Investigation of penetration force of living cell using an atomic force microscope. J. Mech. Sci. Technol. 23:1932–1938, 2009. https://doi.org/10.1007/s12206-009-0508-z.
Lekka, M., et al. Cancer cell detection in tissue sections using AFM. Arch. Biochem. Biophys. 18:151–156, 2012.
Leong, W. S., et al. Thickness sensing of hMSCs on collagen gel directs stem cell fate. Biochem. Biophys. Res. Commun. 401:287–292, 2010.
Lin, R.-Z., and H.-Y. Chang. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 3:1172–1184, 2008. https://doi.org/10.1002/biot.200700228.
Lutolf, M. P. Integration column: artificial ECM: expanding the cell biology toolbox in 3D. Integr. Biol. 1:235–241, 2009.
Ma, X., et al. Fibers in the extracellular matrix enable long-range stress transmission between cells. Biophys. J. 104:1410–1418, 2013.
Medalsy, I. D., and D. J. Müller. Nanomechanical properties of proteins and membranes depend on loading rate and electrostatic interactions. ACS Nano 7:2642–2650, 2013. https://doi.org/10.1021/nn400015z.
Mehta, G., A. Y. Hsiao, M. Ingram, G. D. Luker, and S. Takayama. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control Release 164:192–204, 2012.
Minchinton, A. I., and I. F. Tannock. Drug penetration in solid tumours. Nat. Rev. Cancer 6:583–592, 2006. https://doi.org/10.1038/nrc1893.
Mouw, J. K., G. Ou, and V. M. Weaver. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 15:771–785, 2014.
Nederman, T., B. Norling, B. Glimelius, J. Carlsson, and U. Brunk. Demonstration of an extracellular matrix in multicellular tumor spheroids. Cancer Res. 44:3090–3097, 1984.
O’Reilly, M. S., et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a lewis lung carcinoma. Cell 79:315–328, 1994.
Pampaloni, F., E. G. Reynaud, and E. H. K. Stelzer. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 8:839–845, 2007. https://doi.org/10.1038/nrm2236.
Paszek, M. J., et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8:241–254, 2005.
Plodinec, M., G. Schweighauser, R. Sütterlin, E. Oberman, and U. Aebi. Morphology and cytoarchitecture regulate nanomechanical properties of tumor spheroids. AFM Cancer Diagn. 7:116–145, 2012.
Pöschl, E., U. Schlötzer-Schrehardt, B. Brachvogel, K. Saito, Y. Ninomiya, and U. Mayer. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131:1619–1628, 2004.
Provenzano, P. P., D. R. Inman, K. W. Eliceiri, and P. J. Keely. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 28:4326–4343, 2009. https://doi.org/10.1038/onc.2009.299.
Rico, F., P. Roca-Cusachs, N. Gavara, R. Farré, M. Rotger, and D. Navajas. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys. Rev. E 72:21914, 2005. https://doi.org/10.1103/PhysRevE.72.021914.
Roeder, B. A., K. Kokini, J. E. Sturgis, J. P. Robinson, and S. L. Voytik-Harbin. Tensile mechanical properties of three-dimensional type i collagen extracellular matrices with varied microstructure. J. Biomech. Eng. 124:214–222, 2002.
Sader, J. E., I. Larson, P. Mulvaney, and L. R. White. Method for the calibration of atomic force microscope cantilevers. Rev. Sci. Instrum. 66:3789–3798, 1995.
Sawhney, A., C. Pathak, and J. Hubbell. Bioerodible hydrogels based on photopolymerized poly (ethylene glycol)-co-poly (alpha-hydroxy acid) diacrylate macromers. Macromolecules 26:518–587, 1993.
Shekhar, M. P. V. Drug resistance: challenges to effective therapy. Curr. Cancer Drug Targets 11:613–623, 2011.
Sokolov, I. Toward the nanoscale study of insect physiology using an atomic force microscopy-based nanostethoscope. MRS Bull. 37:522–527, 2012.
Takagi, J., Y. Yang, J. Liu, J. Wang, and T. A. Springer. Complex between nidogen and laminin fragments reveals a paradigmatic β-propeller interface. Nature 424:969–974, 2003.
Tibbitt, M. W., and K. S. Anseth. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 103:655–663, 2009.
Timpl, R., H. Wiedemann, V. van Delden, H. Furthmayr, and K. Kühn. A network model for the organization of type IV collagen molecules in basement membranes. Eur. J. Biochem. 120:203–211, 1981. https://doi.org/10.1111/j.1432-1033.1981.tb05690.x.
Ushiki, T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 65:109–126, 2002.
Vyas, V., N. Nagarajan, P. Zorlutuna, and B. D. Huey. Nanostethoscopy: atomic force microscopy probe contact force versus measured amplitude of cardiomyocytic contractions. J. Bionanosci. 11:319–322, 2017.
Vyas, V., A. Podestà, and P. Milani. Probing nanoscale interactions on biocompatible cluster-assembled titanium oxide surfaces by atomic force microscopy. J. Nanosci. Nanotechnol. 11:4739–4748, 2011.
Vyas, V., M. Solomon, G. G. M. D’Souza, and B. D. Huey. Dynamic and depth dependent nanomechanical properties of dorsal ruffles in live cells and biopolymeric hydrogels. J. Nanosci. Nanotechnol. 18:1557–1567, 2018.
Wenger, M. P., L. Bozec, M. A. Horton, and P. Mesquida. Mechanical properties of collagen fibrils. Biophys. J. 93:1255–1263, 2007.
Winer, J. P., S. Oake, and P. A. Janmey. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLoS ONE 4:e6382, 2009.
Yang, Y. L., L. M. Leone, and L. J. Kaufman. Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys. J. 97:2051–2060, 2009.
Yurchenco, P. D., and B. L. Patton. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 15:1277–1294, 2009.
Yurchenco, P. D., and J. C. Schittny. Molecular architecture of basement membranes. FASEB J. 4:1577–1590, 1990.
Author Contributions
Conceived and designed the experiments: VV BDH. Performed the experiments: VV. Analyzed the data: VV BDH. Contributed biological material & cells: MS GGMD. Wrote the paper: VV.
Conflict of interest
Varun Vyas, Melani Solomon, Gerard G.M. D’Souza and Bryan D. Huey declare that they have no conflicts of interest.
Ethical Approval
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Funding
Provided by the University of Connecticut’s Provosts Fund and NSF Nano-Bio-Mechanics grant 0626231.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Kris Noel Dahl oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vyas, V., Solomon, M., D’Souza, G.G.M. et al. Nanomechanical Analysis of Extracellular Matrix and Cells in Multicellular Spheroids. Cel. Mol. Bioeng. 12, 203–214 (2019). https://doi.org/10.1007/s12195-019-00577-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-019-00577-0