Abstract
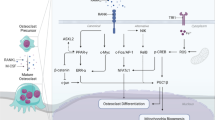
Osteoclasts are bone-resorbing cells that play an essential role in the remodeling of the bone. Defects in osteoclasts thus result in unbalanced bone remodeling, leading to numerous pathological conditions such as osteoporosis, bone metastasis, and inflammatory bone erosion. Metabolism is any process a cell utilizes to meet its energetic demand for biological functions. Along with signaling pathways and osteoclast-specific gene expression programs, osteoclast differentiation activates metabolic programs. The energy generated from metabolic reprogramming in osteoclasts not only supports the phenotypic changes from mononuclear precursor cells to multinuclear osteoclasts, but also facilitates bone resorption, a major function of terminally differentiated, mature osteoclasts. While oxidative phosphorylation is studied as a major metabolic pathway that fulfills the energy demands of osteoclasts, all metabolic pathways are closely interconnected. Therefore, it remains important to understand the various aspects of osteoclast metabolism, including the roles and effects of glycolysis, glutaminolysis, fatty acid synthesis, and fatty acid oxidation. Targeting the pathways associated with metabolic reprogramming has shown beneficial effects on pathological conditions. As a result, it is clear that a deeper understanding of metabolic regulation in osteoclasts will offer broader translational potential for the treatment of human bone disorders.


Similar content being viewed by others
References
Ikeda K, Takeshita S (2016) The role of osteoclast differentiation and function in skeletal homeostasis. J Biochem 159(1):1–8
Nakashima T, Hayashi M, Takayanagi H (2012) New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab 23(11):582–590
Boyce BF (2013) Advances in osteoclast biology reveal potential new drug targets and new roles for osteoclasts. J Bone Miner Res 28(4):711–722
Fuller K, Kirstein B, Chambers TJ (2007) Regulation and enzymatic basis of bone resorption by human osteoclasts. Clin Sci (Lond) 112(11):567–575
Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4(8):638–649
Feldenberg LR, Thevananther S, del Rio M, de Leon M, Devarajan P (1999) Partial ATP depletion induces Fas- and caspase-mediated apoptosis in MDCK cells. Am J Phys 276(6):F837–F846
Spinelli JB, Haigis MC (2018) The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol 20(7):745–754
Eisner V, Picard M, Hajnoczky G (2018) Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat Cell Biol 20(7):755–765
Dudley HR, Spiro D (1961) The fine structure of bone cells. J Biophys Biochem Cytol 11(3):627–649
Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ, Vidal-Puig A, Ikeda K (2009) Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15(3):259–266
Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y (2010) PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab 11(6):503–516
Wan Y (2010) PPARgamma in bone homeostasis. Trends Endocrinol Metab 21(12):722–728
Wei W, Schwaid AG, Wang X, Wang X, Chen S, Chu Q, Saghatelian A, Wan Y (2016) Ligand activation of ERRalpha by cholesterol mediates statin and bisphosphonate effects. Cell Metab 23:479–491
Zeng R, Faccio R, Novack DV (2015) Alternative NF-kappaB regulates RANKL-induced osteoclast differentiation and mitochondrial biogenesis via independent mechanisms. J Bone Miner Res 30(12):2287–2299
Izawa T, Rohatgi N, Fukunaga T, Wang QT, Silva MJ, Gardner MJ, McDaniel ML, Abumrad NA, Semenkovich CF, Teitelbaum SL, Zou W (2015) ASXL2 regulates glucose, Lipid, and Skeletal Homeostasis. Cell Rep 11(10):1625–1637
Wilson L, Yang Q, Szustakowski JD, Gullicksen PS, Halse R (2007) Pyruvate induces mitochondrial biogenesis by a PGC-1 alpha-independent mechanism. Am J Phys Cell Phys 292(5):C1599–C1605
Drosatos-Tampakaki Z, Drosatos K, Siegelin Y, Gong S, Khan S, Van Dyke T, Goldberg IJ, Schulze PC, Schulze-Spate U (2014) Palmitic acid and DGAT1 deficiency enhance osteoclastogenesis, while oleic acid-induced triglyceride formation prevents it. J Bone Miner Res 29(5):1183–1195
Falkenberg M, Gaspari M, Rantanen A, Trifunovic A, Larsson NG, Gustafsson CM (2002) Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat Genet 31(3):289–294
Miyazaki T, Iwasawa M, Nakashima T, Mori S, Shigemoto K, Nakamura H, Katagiri H, Takayanagi H, Tanaka S (2012) Intracellular and extracellular ATP coordinately regulate the inverse correlation between osteoclast survival and bone resorption. J Biol Chem 287(45):37808–37823
Bae S, Lee MJ, Mun SH, Giannopoulou EG, Yong-Gonzalez V, Cross JR, Murata K, Giguere V, van der Meulen M, Park-Min KH (2017) MYC-dependent oxidative metabolism regulates osteoclastogenesis via nuclear receptor ERRalpha. J Clin Invest 127(7):2555–2568
Nishikawa K, Iwamoto Y, Kobayashi Y, Katsuoka F, Kawaguchi S, Tsujita T, Nakamura T, Kato S, Yamamoto M, Takayanagi H, Ishii M (2015) DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine-producing metabolic pathway. Nat Med 21(3):281–287
Lemma S, Sboarina M, Porporato PE, Zini N, Sonveaux P, Di Pompo G, Baldini N, Avnet S (2016) Energy metabolism in osteoclast formation and activity. Int J Biochem Cell Biol 79:168–180
Jin Z, Wei W, Yang M, Du Y, Wan Y (2014) Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab 20(3):483–498
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27:441–464
Karner CM, Long F (2018) Glucose metabolism in bone. Bone 115:2–7
Indo Y, Takeshita S, Ishii KA, Hoshii T, Aburatani H, Hirao A, Ikeda K (2013) Metabolic regulation of osteoclast differentiation and function. J Bone Miner Res 28(11):2392–2399
Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB (2015) Transport of sugars. Annu Rev Biochem 84:865–894
Palazon A, Goldrath AW, Nizet V, Johnson RS (2014) Review HIF transcription factors, inflammation, and immunity. Immunity 41:518–528
Murata K, Fang C, Terao C, Giannopoulou EG, Lee YJ, Lee MJ, Mun SH, Bae S, Qiao Y, Yuan R, Furu M, Ito H, Ohmura K, Matsuda S, Mimori T, Matsuda F, Park-Min KH, Ivashkiv LB (2017) Hypoxia-sensitive COMMD1 integrates signaling and cellular metabolism in human macrophages and suppresses osteoclastogenesis. Immunity 47(1):66–79 e5
Miyauchi Y, Sato Y, Kobayashi T, Yoshida S, Mori T, Kanagawa H, Katsuyama E, Fujie A, Hao W, Miyamoto K, Tando T, Morioka H, Matsumoto M, Chambon P, Johnson RS, Kato S, Toyama Y, Miyamoto T (2013) HIF1alpha is required for osteoclast activation by estrogen deficiency in postmenopausal osteoporosis. Proc Natl Acad Sci U S A 110(41):16568–16573
Knowles HJ, Athanasou NA (2009) Acute hypoxia and osteoclast activity: a balance between enhanced resorption and increased apoptosis. J Pathol 218:256–264
Knowles HJ, Cleton-Jansen A-M, Korsching E, Athanasou NA (2010) Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: role of angiopoietin-like 4. FASEB J 24:4648–4659
Morten KJ, Badder L, Knowles HJ (2013) Differential regulation of HIF-mediated pathways increases mitochondrial metabolism and ATP production in hypoxic osteoclasts. J Pathol 229:755–764
Miyauchi Y, Sato Y, Kobayashi T, Yoshida S, Mori T, Kanagawa H, Katsuyama E, Fujie A, Hao W, Miyamoto K, Tando T, Morioka H, Matsumoto M, Chambon P, Johnson RS, Kato S, Toyama Y, Miyamoto T (2013) HIF1 α is required for osteoclast activation by estrogen de fi ciency in postmenopausal osteoporosis. Proc Natl Acad Sci 110:16568–16573
Leger AJ, Altobelli A, Mosquea LM, Belanger AJ, Song A, Cheng SH, Jiang C, Yew NS (2010) Inhibition of osteoclastogenesis by prolyl hydroxylase inhibitor dimethyloxallyl glycine. J Bone Miner Metab 28(5):510–519
Denko NC (2008) Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 8(9):705–713
Ahn H, Lee K, Kim JM, Kwon SH, Lee SH, Lee SY, Jeong D (2016) Accelerated lactate dehydrogenase activity potentiates osteoclastogenesis via NFATc1 signaling. PLoS One 11(4):e0153886
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, Metabolism, and Disease. Cell 168(6):960–976
Chen J, Long F (2018) mTOR signaling in skeletal development and disease. Bone Res 6:1
Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA (2003) M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ 10(10):1165–1177
Tiedemann K, Le Nihouannen D, Fong JE, Hussein O, Barralet JE, Komarova SV (2017) Regulation of osteoclast growth and fusion by mTOR/raptor and mTOR/rictor/Akt. Front Cell Dev Biol 5:54
Huynh H, Wan Y (2018) mTORC1 impedes osteoclast differentiation via calcineurin and NFATc1. Commun Biol 1:29
Dai Q, Xie F, Han Y, Ma X, Zhou S, Jiang L, Zou W, Wang J (2017) Inactivation of regulatory-associated protein of mTOR (raptor)/mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. J Biol Chem 292(1):196–204
Wu H, Wu Z, Li P, Cong Q, Chen R, Xu W, Biswas S, Liu H, Xia X, Li S, Hu W, Zhang Z, Habib SL, Zhang L, Zou J, Zhang H, Zhang W, Li B (2017) Bone size and quality regulation: concerted actions of mTOR in mesenchymal stromal cells and osteoclasts. Stem Cell Reports 8(6):1600–1616
Tintut Y, Morony S, Demer LL (2004) Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol 24(2):e6–e10
Kim SP, Li Z, Zoch ML, Frey JL, Bowman CE, Kushwaha P, Ryan KA, Goh BC, Scafidi S, Pickett JE, Faugere MC, Kershaw EE, Thorek DLJ, Clemens TL, Wolfgang MJ, Riddle RC (2017) Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight 2(16)
D.M.A. P.K.LUND, and J.C. MATHI, Lipid composition of normal human bone marrow as determined by column chromatography, Journal of Lipid Resesrch 3(1) (1962)
Kasonga AE, Deepak V, Kruger MC, Coetzee M (2015) Arachidonic acid and docosahexaenoic acid suppress osteoclast formation and activity in human CD14+ monocytes, in vitro. PLoS One 10(4):e0125145
Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G (2003) Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res 18(7):1206–1216
Mangano KM, Sahni S, Kerstetter JE, Kenny AM, Hannan MT (2013) Polyunsaturated fatty acids and their relation with bone and muscle health in adults. Curr Osteoporos Rep 11(3):203–212
Oh SR, Sul OJ, Kim YY, Kim HJ, Yu R, Suh JH, Choi HS (2010) Saturated fatty acids enhance osteoclast survival. J Lipid Res 51(5):892–899
Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R (2016) Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 126(6):2049–2063
Correa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA (2016) Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol 5(4):e73
Lucas S, Omata Y, Hofmann J, Bottcher M, Iljazovic A, Sarter K, Albrecht O, Schulz O, Krishnacoumar B, Kronke G, Herrmann M, Mougiakakos D, Strowig T, Schett G, Zaiss MM (2018) Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun 9(1):55
Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS (2008) Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab 8(6):512–521
Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G (1999) Stimulation of bone formation in vitro and in rodents by statins. Science 286(5446):1946–1949
Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV Jr (2000) Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25(1):87–90
Sato T, Morita I, Murota S (1998) Involvement of cholesterol in osteoclast-like cell formation via cellular fusion. Bone 23(2):135–140
Okayasu M, Nakayachi M, Hayashida C, Ito J, Kaneda T, Masuhara M, Suda N, Sato T, Hakeda Y (2012) Low-density lipoprotein receptor deficiency causes impaired osteoclastogenesis and increased bone mass in mice because of defect in osteoclastic cell-cell fusion. J Biol Chem 287(23):19229–19241
Luegmayr E, Glantschnig H, Wesolowski GA, Gentile MA, Fisher JE, Rodan GA, Reszka AA (2004) Osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins. Cell Death Differ 11(Suppl 1):S108–S118
Ryu J, Kim H, Chang EJ, Kim HJ, Lee Y, Kim HH (2010) Proteomic analysis of osteoclast lipid rafts: the role of the integrity of lipid rafts on V-ATPase activity in osteoclasts. J Bone Miner Metab 28(4):410–417
Wellen KE, Thompson CB (2012) A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol 13(4):270–276
Acknowledgements
Figures are created with BioRender.com.
Funding
This work was supported by grants from the N.I.H. (R01AR069562 and R01 AR073156-01), by Weill Cornell CTSC, and by support for the Rosensweig Genomics Center from The Tow Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is a contribution to the special issue on Osteoimmunology - Guest Editor: Mary Nakamura
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park-Min, KH. Metabolic reprogramming in osteoclasts. Semin Immunopathol 41, 565–572 (2019). https://doi.org/10.1007/s00281-019-00757-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-019-00757-0