Abstract
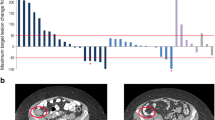
This multicenter, phase I, open-label dose escalation study evaluated safety, tolerability, pharmacokinetics, and preliminary anti-tumor activity of inebilizumab in Japanese patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), chronic lymphocytic leukemia (CLL), follicular lymphoma (FL), or multiple myeloma (MM) who were ineligible for hematopoietic stem cell transplantation. Patients received inebilizumab 2, 4, or 8 mg/kg intravenously on days 1 and 8 of the first 28-day cycle, and once every 28 days thereafter, with a 12 mg/kg cohort added. Twenty patients (11 FL, six DLBCL, two CLL, and one MM) received inebilizumab at four dose levels (2 mg/kg cohort, n = 3; 4 mg/kg cohort, n = 7; 8 mg/kg cohort, n = 4; 12 mg/kg cohort, n = 6). Three patients experienced dose-limiting toxicities: grade 4 neutropenia/grade 3 leukopenia (n = 1, 12 mg/kg) and grade 3 infusion reaction (n = 1 each, 4 mg/kg and 12 mg/kg); the maximum tolerated dose was 8 mg/kg. Four (three FL and one DLBCL) patients achieved complete response; eight (six FL and two DLBCL) achieved partial response. Overall response rate was 60%. Over the dose ranges evaluated, the pharmacokinetic profile of inebilizumab in Japanese patients was generally dose proportional. This phase I study showed acceptable toxicity and preliminary and promising efficacy of inebilizumab in patients with relapsed/refractory FL and DLBCL.


Similar content being viewed by others
References
Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380:848–57.
Nastoupil LJ, Rose AC, Flowers CR. Diffuse large B-cell lymphoma: current treatment approaches. Oncology (Williston Park). 2012;26:488–95.
Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematol Am Soc Hematol Educ Program. 2011;2011:498–505.
Hennessy BT, Hanrahan EO, Daly PA. Non-Hodgkin lymphoma: an update. Lancet Oncol. 2004;5:341–53.
Igarashi T, Ogura M, Itoh K, Taniwaki M, Ando K, Kuroda Y, et al. Japanese phase II study Of rituximab maintenance for untreated indolent b-cell non-hodgkin lymphoma with high tumor burden. Int J Hematol. 2016;104:700–708 [Erratum in: Igarashi T, Ogura M, Itoh K, et al. Int J Hematol. 2017;105:109–110].
Robak T, Stilgenbauer S, Tedeschi A. Front line treatment of CLL in the era of novel agents. Cancer Treat Rev. 2017;53:70–8.
Galaznik A, Huelin R, Stokes M, Guo Y, Hoog M, Bhagnani T, et al. Systematic review of therapy used in relapsed or refractory diffuse large B-cell lymphoma and follicular lymphoma. Future Sci OA. 2018;4:FSO322.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17.
Usmani S, Ahmadi T, Ng Y, Lam A, Desai A, Potluri R, et al. Analysis of real-world data on overall survival in multiple myeloma patients with ≥ 3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or double refractory to a PI and an IMiD. Oncologist. 2016;21:1355–61.
Cooper LJ, Al-Kadhimi Z, DiGiusto D, Kalos M, Colcher D, Raubitschek A, et al. Development and application of CD19-specific T cells for adoptive immunotherapy of B cell malignancies. Blood Cells Mol Dis. 2004;33:83–9.
Ginaldi L, De Martinis M, Matutes E, Farahat N, Morilla R, Catovsky D. Levels of expression of CD19 and CD20 in chronic B cell leukaemias. J Clin Pathol. 1998;51:364–9.
Uckun FM, Jaszcz W, Ambrus JL, Fauci AS, Gajl-Peczalska K, Song CW, et al. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988;71:13–29.
Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984;63:1424–33.
D’Arena G, Musto P, Cascavilla N, Dell’Olio M, Di RN, Carotenuto M. Quantitative flow cytometry for the differential diagnosis of leukemic B-cell chronic lymphoproliferative disorders. Am J Hematol. 2000;64:275–81.
Rasmussen T, Jensen L, Johnsen HE. The CD19 compartment in myeloma includes a population of clonal cells persistent after high-dose treatment. Leuk Lymphoma. 2002;43:1075–7.
Bergsagel PL, Smith AM, Szczepek A, Mant MJ, Belch AR, Pilarski LM. In multiple myeloma, clonotypic B lymphocytes are detectable among CD19 + peripheral blood cells expressing CD38, CD56, and monotypic Ig light chain. Blood. 1995;85:436–47.
Kiel K, Cremer FW, Rottenburger C, Kallmeyer C, Ehrbrecht E, Atzberger A, et al. Analysis of circulating tumor cells in patients with multiple myeloma during the course of high-dose therapy with peripheral blood stem cell transplantation. Bone Marrow Transplant. 1999;23:1019–27.
Huff CA, Matsui W. Multiple myeloma cancer stem cells. J Clin Oncol. 2008;26:2895–900.
Herbst R, Wang Y, Gallagher S, Mittereder N, Kuta E, Damschroder M, et al. B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. J Pharmacol Exp Ther. 2010;335:213–22.
Ward E, Mittereder N, Kuta E, Sims GP, Bowen MA, Dall’Acqua W, et al. A glycoengineered anti-CD19 antibody with potent antibody-dependent cellular cytotoxicity activity in vitro and lymphoma growth inhibition in vivo. Br J Haematol. 2011;155:426–37.
Gallagher S, Turman S, Yusuf I, Akhgar A, Wu Y, Roskos LK, et al. Pharmacological profile of MEDI-551, a novel anti-CD19 antibody, in human CD19 transgenic mice. Int Immunopharmacol. 2016;36:205–12.
Forero-Torres A, Hamadani M, Fanale MA, Bello CM, Kipps TJ, Offner F, et al. Safety, profile and clinical response to MEDI-551, a humanized monoclonal anti-CD19, in a phase 1/2 study in adults with relapsed or refractory advanced B-cell malignancies. Blood. 2013;122:1810. (abstract).
Boucher K, Parquet N, Widen R, Shain K, Baz R, Alsina M, et al. Stemness of B cell progenitors in multiple myeloma bone marrow. Clin Cancer Res. 2012;18:6155–68.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008; 111:5446–56.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Zhang K, Wang F, Zhang M, Cao X, Yang S, Jia S, et al. Reference ranges of lymphocyte subsets balanced for age and gender from a population of healthy adults in Chongqing District of China. Cytom Part B. 2016;90B:538–42.
Valiathan R, Deeb K, Diamante M, Ashman M, Sachdeva N, Asthana D. Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology. 2014;219:487–96.
Ribrag V, Dupuis J, Tilly H, Morschhauser F, Laine F, Houot R, et al. A dose-escalation study of SAR3419, an anti-CD19 antibody maytansinoid conjugate, administered by intravenous infusion once weekly in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2014;20:213–20.
Acknowledgements
The authors thank the patients who participated in this study and their families. We also thank all the investigators, nursing staff, and research support staff who contributed to the study. Medical writing and editorial support were provided by Amy Zannikos, PharmD, of Peloton Advantage (Parsippany, NJ) and funded by MedImmune, the global biologics R&D arm of AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.O., M.O., Y.S., K.A., T.U., I.C., Y.O., M.K. and J.O. have received research Grants from AstraZeneca. K.F. was an employee of AstraZeneca and owned stock/stock options in the company at the time of the study. Y.Y. is an employee of AstraZeneca and owns stock/stock options in the company. This study was designed under the responsibility of MedImmune, the global biologics R&D arm of AstraZeneca, the study was funded by MedImmune, and the study drug was provided by MedImmune. Each author had full access to the data, made substantial contributions to the drafting and/or revision of this manuscript, and granted approval for the submission of this paper. No author received an honorarium or other form of financial support related to the development of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ohmachi, K., Ogura, M., Suehiro, Y. et al. A multicenter phase I study of inebilizumab, a humanized anti-CD19 monoclonal antibody, in Japanese patients with relapsed or refractory B-cell lymphoma and multiple myeloma. Int J Hematol 109, 657–664 (2019). https://doi.org/10.1007/s12185-019-02635-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02635-9