Abstract
The natriuretic peptide (NP) system comprises of three ligands, the Atrial Natriuretic Peptide (ANP), Brain Natriuretic peptide (BNP) and C-type Natriuretic peptide (CNP), and three natriuretic peptide receptors, NPRA, NPRB and NPRC. Here we present a comprehensive study of the natriuretic peptide system in healthy murine and human submandibular salivary glands (SMGs). We show CNP is the dominant NP in mouse and human SMG and is expressed together with NP receptors in ducts, autonomic nerves and the microvasculature of the gland, suggesting CNP autocrine signalling may take place in some of these glandular structures. These data suggest the NP system may control salivary gland function during homeostasis through the regulation of electrolyte re-absorption, neural stimulation and/or blood vessel wall contraction/relaxation. We also show abnormal expression of NPRA in the stroma of a subset of human SMGs resected from patients diagnosed with oral squamous cell carcinoma (OSCC) of non-salivary gland origin. This finding warrants further research to investigate a possible correlation between early OSCC invasion and NPRA overexpression.
Similar content being viewed by others
Introduction
The natriuretic peptide (NP) system is composed of three ligands—Atrial Natriuretic Peptide (ANP), Brain Natriuretic Peptide (BNP) and C-type Natriuretic Peptide (CNP)—and three natriuretic peptide receptors (NPRs), NPRA, NPRB and NPRC. NPs have been shown to bind to NPRs selectively. ANP and BNP bind to NPRA and NPRC, while CNP binds to NPRB and NPRC (Koller et al. 1991; Suga et al. 1992a). NPRA and NPRB are guanylyl cyclase receptors mediating the production of cGMP, which regulates a number of intracellular signalling pathways in a complex manner. NPRC lacks a catalytic domain and therefore has a different mode of action. NPRC, which binds to all NPs with a similar affinity, was initially implicated in the metabolic clearance of NPs. Following the binding of NPs to NPRC, the ligand/receptor complexes are internalized, with the ligands undergoing lysosomal degradation, while the NPRC receptor is recycled to the plasma membrane (Potter 2011). Apart from its role in NP clearance, NPRC is also coupled to Gi-dependent signalling leading to adenylyl cyclase inhibition and a subsequent reduction in intracellular cAMP levels in certain tissues (Pagano and Anand-Srivastava 2001; Murthy et al. 2000).
ANP is predominantly produced in the atrial myocardium, BNP in the brain and ventricular myocardium and CNP in the central nervous system and vascular endothelium (Suga et al. 1992b). ANP and BNP have endocrine, paracrine and autocrine effects (D'Souza et al. 2004; Evrard et al. 1999), whereas CNP appears to act as local paracrine regulator (Suda et al. 1996; Suga et al. 1993). The best characterised function of the NP system relates to the control of the pressure–volume homeostasis of the renal-cardiovascular system. Upon stretch of the cardiac wall, ANP and BNP are released into the circulation, inducing increased renal excretion of sodium and water, increased vascular permeability and vasodilation, leading to a reduction in systemic blood pressure (Curry 2005; Houben et al. 2005), while CNP induces vasodilation (Wei et al. 1993). Recent research has also shown BNP and CNP regulate cardiovascular function by direct inhibition of cardiac sympathetic neurotransmission (Buttgereit et al. 2016; Li et al. 2015). Identification of NP production and NPR expression in a broad range of tissues suggests this system has biological effects beyond the regulation of kidney and cardiovascular functions. Immunoreactivity for ANP has been identified in the brain, ganglia of the peripheral nervous system, spinal cord, pituitary gland, eye, lung, adrenal medulla, thymus and thyroid gland (Gutkowska and Nemer 1989; Vollmar 1990; Hodes and Lichtstein 2014). Furthermore, NPRs are present in a large number of cell and tissue types. For example, macrophages, T-lymphocytes and dendritic cells express receptors for ANP (Kiemer and Vollmar 2001; Mohapatra 2007) and there is growing evidence the NP system plays a significant role in the modulation of the immune system. Indeed, mice deficient for NprA, the receptor for ANP, exhibit reduced lung inflammation in an injury model for allergic asthma, indicating ANP/NprA signalling plays a significant role in the inflammatory process (Kong et al. 2008). Importantly, NP and NPR levels are altered in pathophysiological conditions such as heart failure (Volpe et al. 2016) and cancers (Li et al. 2016; Zhao et al. 2014; Wang et al. 2011). Hence understanding the varied physiological effects of NP signalling and the mechanisms at play can help elucidate the role and diagnostic and therapeutic potential of the NP system in pathophysiological conditions.
The presence of NPs has been reported in salivary glands (SGs). There are three pairs of major SGs, the parotid, sublingual and submandibular salivary glands, producing respectively a serous, mucous and mixed saliva. SGs contain secretory acinar cells organised in units (acini) that empty their secretions in ducts of increasing diameters, formed of intercalated ducts, granular ducts (rodent-specific), striated ducts and excretory ducts, by order of increasing diameter. A primary saliva produced in acini is modified as it passes through striated ducts. Na+ and Cl− ions are reabsorbed while K+ and HCO3− ions are secreted, resulting in the production of a saliva hypotonic to plasma. The secretory function of SGs is under control of the autonomic nervous system (ANS). The two divisions of the ANS have very distinctive effects on saliva production: the stimulation of parasympathetic fibres leads to the production of watery secretions, while sympathetic stimulation induces protein-rich viscous secretions. Immunoreactivity for ANP and CNP was identified in the intercalated, granular and striated ducts as well as in endothelial cells of rat parotid and submandibular glands (Gutkowska and Nemer 1989; Cantin et al. 1984; Vollmar et al. 1991; Cho et al. 2000a). Moreover, identification of Nppa mRNA (encoding ANP) and ANP prohormone (a storage form of ANP) in rat parotid glands further suggested ANP was locally synthesized in SGs (Vollmar et al. 1991). Interestingly, immunoreactivity for ANP in adult parotid gland ducts is conserved in humans (Lipari et al. 2013). ANP levels in healthy human saliva correlate with plasma levels (Gauquelin et al. 1992), suggesting either ANP is secreted in saliva but produced in small amounts in SGs, or ANP locally produced in SGs is not secreted in saliva. While the three NP receptors were found to be expressed in rat parotid glands (Nashida et al. 1996), the spatial distribution of these receptors, which could have suggested a local role for the NP system in SGs, was not investigated. We have carried out a comprehensive study of the expression and spatial distribution of all NPs and their receptors in the adult submandibular gland (SMG), a major SG commonly used as a model to study SG physiological and pathophysiological states. Our investigation reveals the dominant NP expressed in mouse and human SMGs is CNP. CNP is expressed in autonomic nerves, ducts and blood vessels of the human SMG together with the three NPRs, which offers the possibility CNP autocrine signaling may take place in distinct SG structures. The NP system may therefore regulate SG function by controlling autonomic nerve stimulation, blood vessel diameter and/or saliva composition. We show components of the NP system exhibit mostly similar distributions in murine and human SMGs apart from microvasculature expression of the NP system that is specific to human SMGs. We also analyzed human SMG tissues obtained from patients diagnosed with oral squamous cell carcinomas (OSCC). Although these SMG tissues did not show gross morphological changes, ectopic NPRA expression was identified in the glandular stroma of a subset of OSCC cases. Considering NPRA overexpression has been identified in a number of malignancies (Li et al. 2016; Zhao et al. 2014; Wang et al. 2011), this finding warrants further studies to explore a potential link between early OSCC invasion and NPRA overexpression.
Materials and methods
Ethics statement
The care and use of all mice in this study were conducted in accordance with the United Kingdom’s Animal (Scientific Procedures) Act of 1986, under two UK project licences (70/7866 and P5F0A1579), which were approved by the UK Home Office. Human salivary gland tissues were obtained from patients who had undergone surgery at the Division of Surgery of the Medical School within the University of Palermo (Italy). Informed consent was obtained from all patients. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Salivary gland tissue
CD1 wild-type adult mice over 8 weeks-old were used. Submandibular salivary glands (SMGs) were excised, fixed in 4% paraformaldehyde and embedded in paraffin wax. Human SMG tissues embedded in paraffin were from the archives of the Department of Pathology of the University of Palermo. Human SMG biopsies from eight patients were analyzed, comprising three normal SMGs and five SMGs resected from patients diagnosed with squamous cell carcinomas (SCCs) of non-SG origin. Tumor cases presented a history of variable size of primary tumors as well as variable presence of metastasis in draining lymph nodes. Cases of SCCs analyzed originated from various locations within the oral cavity, including the lower lip, larynx and tongue. Human SMG biopsies were included in the present study after being reviewed by a pathologist who confirmed the initial diagnosis. The median age of SMG biopsies analyzed in this study was 58.5 years (36–67 years).
Immunostaining
4–7 µm-thick tissue sections were de-paraffinized and rehydrated. For immunohistochemistry, an antigen retrieval was performed with the Novocastra Epitope Retrieval Solutions pH 6 and pH 9 (Leica) in a PT Link station (Agilent). After neutralization of endogenous peroxidase with 3% H2O2 and incubation in blocking solution (Novocastra UK), tissue sections were incubated overnight with the following primary antibodies: mouse anti-ANP (1:50, #ab2093, Abcam); rabbit anti-NPRA (1:100, #orb38973, Biorbyt), rabbit anti-BNP (1:500, #ab19645, Abcam), rabbit anti-pro-CNP (1:100, #ab202139, Abcam), rabbit anti-NPRA (1:50, #ab70848, Abcam), rabbit anti-NPRB (1:100, #ab14357Abcam), rabbit anti-NPRC (1:50, #ab37617, Abcam), mouse anti-beta III tubulin (1:100, #ab78078, Abcam). The Novocastra Novolink™ detection system (Leica) based on biotin-free Compact Polymer™ was used to increase detection sensitivity. Slides were counterstained with Harris hematoxylin (Novocastra), coverslipped with Aquatex (Merck) (Manzel-Glaser) and imaged with a Nikon (Y-THPL) microscope. For immunohistofluorescence, antigen retrieval was performed in a pressure cooker (Biocare Medical) with a pH 9 citrate Target Retrieval Solution (Dako). Tissues were permeabilized with 0.05% Tween twice for 5 min, blocked for 1 h in 1X PBS containing 10% Goat serum, 1% BSA and 0.05% Triton®, and then incubated overnight at 4 °C with previously described primary antibodies in 1X PBS with 1% Goat serum, 1% BSA and 0.05% Triton®. The next day, tissue sections were washed in 1X PBS with 0.1% Triton for three times 5 min, followed by incubation in secondary antibodies (1:200, #DI-2488, DyLight 488 goat anti-mouse and 1:200, #DI-1594, DyLight 594 Goat anti-rabbit, Vector) for 1 h at room temperature. Nuclei were counterstained with 1:500 Hoechst (Sigma). Slides were then coverslipped with CitiFluor® (Electron Microscopy Sciences) mounting medium. Fluorescence was visualized with a Leica TCS SP5 confocal laser microscope.
RNA extraction, RT-qPCR
RNA was extracted from adult wild-type CD1 mouse SMGs using Trizol (Ambion). The extracted RNA was further purified using the DNA-free™ Kit (Ambion) and quantified using a Nanodrop 2000 spectrometer (Thermo Scientific). Complementary DNA (cDNA) was made by reverse transcription of extracted RNA using random primers (M-MLV Reverse Transcriptase kit, Promega) following the manufacturer’s instructions. 384-well qPCR plates (BioRAD, HSR480) were used to assay gene expression by real-time qPCR using a SYBR Green master mix (PowerUp™ SYBR™ Green Master Mix) on a BioRAD CFX384 Touch™ Real-Time PCR Detection System. Mouse primer sequences are listed in Table 1. GAPDH was used to normalize the relative quantification of amplified genes and Ct values were calculated by the 2−ΔΔCT method (Livak and Schmittgen 2001). This analysis was conducted using three SMGs from three distinct animals. For human biopsies, RNA was extracted using the RNeasy FFPE kit (Qiagen) from 4 paraffin-embedded tissue sections of 5 μm each, following the manufacturer's instructions. Purified RNA was quantified using the Qubit 3.0 fluorometer (Life technology). cDNA synthesis was carried out with the Quantinova reverse transcripton Kit (Qiagen) following the manufacturer's instructions. Real-time quantitative polymerase chain reactions (qPCR) were performed with the Quantinova SYBR green PCR kit (Qiagen) in triplicate on a Rotor-Gene Q system (Qiagen) following the manufacturer's instructions. QuantiTech Primer assay (Qiagen) was used with Hs_NPPA_1_SG QT00203322, and Hs_ACTB_1_SG QT00095431 primers, for the quantification of human NPPA and ACTB (normalizer) transcripts, respectively. Data were analyzed using the 2−ΔΔCT method.
Results
All natriuretic system components, except BNP, are locally expressed in mouse submandibular glands
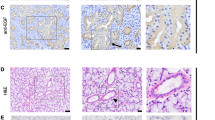
In order to identify which ligands and receptors of the NP system were locally expressed in SGs, we first studied their RNA expression levels in adult mouse submandibular glands (mSMGs). Primers were initially validated with RNA extracted from mouse adult heart and brain tissues. All primers amplified products of expected size in both tissues (data not shown). In the mSMG, we found all three receptors were expressed, with Npr1 (encoding NprA) displaying highest levels of expression, followed by Npr2 (encoding NprB) and Npr3 (encoding NprC). Only Nppa and Nppc, the genes encoding respectively ANP and CNP, were expressed (Fig. 1a). Nppa was the least expressed gene, with expression found in only one of the three male mSMGs analysed and the Ct value for Nppa came up late at cycle 37 out of 40. Both male (Fig. 1a) and female (data not shown) mSMGs showed similar levels of gene expression. Similar to data obtained in males, Nppa expression was identified in only two of the three female mSMGs analysed (data not shown). We next investigated by immunohistochemistry which cells produced NPs and expressed NPRs with antibodies previously validated on mouse heart tissue. With the exception of NprA, regular immunohistochemistry proved not sensitive enough to detect NprB, NprC, ANP and pro-CNP (a propeptide that needs to be cleaved to produce a biologically active CNP) (Wu et al. 2003), suggesting these proteins were present in very low amounts in mSMGs. Immunohistochemistry combined with a polymer amplification system was tested on adult mouse and human SMGs for non-specific immunostaining as background immunostaining has been previously reported in SGs (Cauli et al. 1994). In the absence of primary antibodies, no background staining could be observed in ducts, acini and neurovascular bundles of adult mouse and human SMGs (Fig. 2a–d). However, non-specific staining could be observed occasionally inside mouse and human blood vessels (Fig. 2e, f). Immunohistochemistry combined with polymer amplification revealed the three NPRs were expressed in a subset of granular ducts cells (Fig. 1b–f), as well as in autonomic nerves (Fig. 1g–p). In mice, the submandibular and sublingual SGs are located within the same capsule and a chain of parasympathetic glanglia is found at the hilum of the glands, a region containing large neurovascular bundles (Fig. 1k, p). Immunoreactivity for NprA, NprB and NprC was found in neurons populating parasympathetic submandibular ganglia (Fig. 1g–i) and large nerve bundles (Fig. 1g–i, l–n), while immunoreactivity for NprA and NprC was found in the muscular walls of arterioles (Fig. 1l–n). We took advantage of the high expression level of NprA in mSMG to further investigate by double immunofluorescence whether this NPR was present in neural cells. Double immunofluorescence for NprA and beta III tubulin showed high levels of NprA in nerve bundles, which co-localized with beta III tubulin, confirming NprA was expressed in axons (Fig. 1q1–q3). Lower levels of NprA were identified in ganglionic neuron cell bodies (Fig. 1r1–3r). NprA immunoreactivity was notably mainly found in the cytoplasm of granular duct cells and parasympathetic ganglion neurons. Both pro-CNP and ANP were identified in ducts. However, while ANP immunoreactivity was found in a subset of granular duct cells (Fig. 1f), pro-CNP appeared to be specifically produced by striated duct cells (Fig. 1e). This finding was confirmed in sublingual salivary glands. Mouse sublingual glands, which do not contain granular ducts, presented strong immunoreactivity for pro-CNP in striated ducts (data not shown). While ANP was not detected outside granular ducts, low immunoreactivity for pro-CNP was also found in parasympathetic ganglia (Fig. 1j). However, although pro-CNP was detected in parasympathetic neuronal cell bodies, pro-CNP immunoreactivity was not found in neuro-vascular bundles (Fig. 1o).
Expression of the natriuretic peptide system in mouse adult submandibular salivary glands. a RT-qPCR for Npr1, Npr2, Npr3, Nppa, Nppb and Nppc encoding respectively the three natriuretic receptors NprA, NprB and NprC and the three natriuretic ligands ANP, BNP and CNP in male mSMGs. The lowest gene expression (Nppa) is represented as one. Expression of other genes was compared to Nppa expression. b–p Immunohistochemistry for NprA (b, g, l), NprB (c, h, m), NprC (d, i, n), CNP (e, j, o), ANP (f) and beta III tubulin (k, p). b–d, f The three receptors and ANP are present in granular ducts. Note a minority of granular duct cells are devoid of immunoreactivity. e Immunostaining for CNP is observed in striated ducts and not granular ducts. An enlargement of a stained striated ducts is presented in the lower right corner showing staining concentrated in the basal striations. g–i The three receptors are present in the submandibular parasympathetic ganglia (g, green arrowheads) as well as large nerve bundles (red arrowheads). l–n Immunoreactivity for natriuretic receptors in big nerve bundles (red arrowheads) as well as the blood vessel walls of arterioles (blue arrowheads). j CNP is the only NP detected in parasympathetic ganglia. However, no immunoreactivity for CNP is found in neurovascular bundles (o). k, p Immunoreactivity for beta III tubulin in parasympathetic ganglia (g), nerve bundles (red arrowheads) and arteriole wall (blue arrowheads). q–r Double immunofluorescence for NprA and beta III tubulin. q1–q3 NprA and beta III tubulin co-localise in axons. r1–r3 NprA and beta III tubulin co-localise in axons (white arrowheads) and ganglionic neuron cell bodies. The scale bar represents 40 µm in b–p and 25 µm in q1–s3. Bv blood vessel, d duct, g ganglion
Non-specific background immunostaining in adult murine and human submandibular salivary gland tissue is restricted to content of blood vessels. All steps of immunodetection were carried out, but primary antibodies were omitted from the first incubation step. Immunostaining with anti-rabbit secondary antibodies are shown here. Similar results were obtained with anti-mouse secondary antibodies. No background immunostaining is observed in mouse (a, yellow outline) and human (b) SMG ducts, as well as in mouse (c) and human (d) neuro-vascular bundles. Some non-specific background immunostaining (yellow arrowheads) could be observed inside the blood vessels of mouse (e) and human (f) SMGs. The scale bars represent 50 µm in all panels. Bv blood vessel, d duct, g ganglion
Expression of the NP system in ducts, autonomic nerves and blood vessels is conserved in human submandibular glands
We next investigated whether the presence of NPs and NPRs was conserved in human submandibular glands (hSMGs). hSMG biopsies archived in paraffin wax were used to perform immunohistochemistry with the same polymer amplification system that was used on mSMG. Immunoreactivity for the three ligands and three receptors was detected in striated ducts (Fig. 3a–f). Since only a subset of mSMGs showed Nppa expression, we investigated expression of NPPA in three normal hSMG biopsies from three independent patients. While the ACTB gene used to normalise mRNA levels between samples was expressed in all hSMG biopsies, one hSMG biopsy did not exhibit NPPA expression (Fig. 3v), suggesting either ANP is not produced in SMG of all individuals or ANP production is differentially regulated. Stronger staining for pro-CNP could be observed in the luminal part of striated duct cells, suggesting CNP may be secreted in saliva (Fig. 3c). While BNP and ANP immunoreactivity was not observed in other hSMG structures (Fig. 3g, h, m, n), pro-CNP was found in nerve bundles (Fig. 3i) as well as in blood vessel walls (Fig. 3o). In autonomic nerve bundles, immunostaining for the three receptors was found in the cell membranes, whereas pro-CNP immunostaining located to cell cytoplasm, in line with their function as receptors and secreted ligand, respectively. Double immunofluorescence for NPRA and beta III tubulin showed co-localisation of NPRA and beta III tubulin in nerve bundles, confirming the NPRA receptor is present in neural cells (Fig. 3t1–t3). In small arterioles, both the inner endothelial lining and beta III tubulin+ adventitia were positive for NPRA (Fig. 3u1–u3). Finally, uniform NPRA immunoreactivity was found in ducts (Fig. 3s1–s3).
All natriuretic peptide components are produced within human submandibular glands. a–r Immunohistochemistry on normal adult hSMGs showing immunoreactivity in striated ducts (a–f), nerve bundles (g–l) and blood vessels (m–r). All natriuretic peptides and receptors are present within SMG striated ducts (a–f), note accumulation of pro-CNP on the luminal side of striated duct cells (c) and presence of pro-CNP in larger interlobular collecting ducts (o). Apart from ANP and BNP (g, h, m, n), immunoreactivity for all natriuretic components is found in nerve bundles (i–l, black arrowheads) as well as in the muscular wall of arterioles (o–r, red arrowheads). s1–u3 Double immunohistofluorescence for NPRA (red) and beta III tubulin (green). Immunoreactivity for NPRA is found in ducts (s1–s3) and nerve bundles, in which it co-localises with beta III tubulin (t1–t3). In blood vessels, NPRA is found within the innermost endothelial cell layer as well as in the beta III tubulin+ outer adventitia layer (u1–u3). v RT-qPCR for NPPA (encoding the natriuretic peptide ANP) on normal hSMGs. NPPA expression was found in two out of the three normal hSMGs analysed. The scale bar represents 40 µm in a–r and 25 µm in s1–u3. Bv blood vessel, cd collecting duct, d duct
Ectopic NPRA expression in submandibular glands of patients with oral squamous cell carcinoma
NPRA overexpression in a number of malignancies (Li et al. 2016; Zhao et al. 2014; Wang et al. 2011) combined with the requirement for NprA for tumour growth in a murine model (Kong et al. 2008) prompted us to study NPRA distribution in SMG tissue in pathological situations. Malignant tumours of SGs are uncommon, with squamous cell carcinoma (SCC) accounting for less than 1% of SG tumours (Kaszuba et al. 2007). Hence, we studied NPRA distribution in hSMG biopsies obtained from patients with cutaneous or mucosal head and neck SCC, who had undergone large tissue resections containing SMG tissue. We examined SCC cases that originated from several different locations in the head and neck region, including the larynx, tongue, lower lip and mucosa of the oral cavity. These SCC cases presented with primary tumours of variable size and metastasis had been identified in regional tumour-draining lymph nodes in all clinical cases studied. Haematoxylin and eosin staining of resected hSMG tissues was reviewed by a pathologist who did not find significant morphological anomalies. Localised inflammatory cell infiltrates were observed in all SMG tissues studied, which comprised of three normal SMG tissues and five SMG samples collected from patients with SCC of non-SG origin. Consistent with previous observations made in normal hSMG tissue (Fig. 4a, d), immunoreactivity for NPRA was observed in striated ducts, blood vessel walls and nerves of all SMG biopsies obtained from head and neck SCC patients. However, in two out of the five cases of head and neck SSC analysed, strong ectopic immunoreactivity for NPRA was identified in the stromal tissue of the SMG, surrounding acini and intra-lobular ducts (Fig. 4b, c, e, f) as well as in the connective tissue of septa, while acinar cells remained devoid of immunoreactivity for NPRA. The NPRA receptor being able to bind with a high affinity to both ANP and BNP (Koller et al. 1991; Suga et al. 1992a), we investigated whether the distribution of these two natriuretic peptides was altered in SMG tissues exhibiting high NPRA expression in the glandular stroma. Both ANP and BNP, as well as NPRB and NPRC, did not show significant changes in expression (data not shown).
Changes in NPRA expression are observed in a subset of human submandibular glands from patients with OSCC from non-SG origin. a–f Immunohistochemistry for NPRA on normal hSMGs from a 62-year-old male (a, d) and hSMGs obtained from two patients with head and neck cutaneous or mucosal squamous cell carcinoma showing ectopic stromal expression of NPRA (b, c, e, f). Head and neck cancer patients showing abnormally high NPRA expression were both males and respectively 60-year-old (b, e) and 57-year-old (c, f). d–f are higher magnifications of areas taken respectively from a, b and c. a, d In normal hSMGs, NPRA immunoreactivity is restricted to striated ducts (d) and blood vessel walls (bv). Acinar cells (Ac) do not express NPRA. b–f Out of five SCC cases analysed, two cases presented ectopic NPRA expression in the stroma of hSMGs. The scale bars represent 50 µm in all panels
Discussion
In this study, we carried out a comprehensive analysis of the NP system in the murine and human adult SMG. In adult hSMGs, we found all NPRs were strongly expressed in ductal cells, nerve bundles and blood vessel walls. CNP was co-expressed with NPRs in ducts, nerves and blood vessels, while ANP and BNP expression was restricted to striated ducts. Although the NP system showed a grossly similar expression pattern in the murine adult SMG, a few differences were observed. Whereas the three NPRs were expressed in ducts, neurons of the submandibular parasympathetic ganglia and large nerve bundles, only NprA and NprC were present in murine blood vessel walls. The lack of NprB in blood vessels was mirrored by the lack of expression of its ligand CNP in the walls of blood vessels. Furthermore, BNP was not expressed in murine SMGs. Despite these species variations, CNP appears as the major NP locally produced in SMGs, both in mice and humans. Interestingly, while previous reports have demonstrated the production of ANP in SGs, our study suggests local production of ANP is marginal in the SMG, being either barely detectable or absent both in mouse and human SMGs, as shown by qPCR, a highly sensitive detection technique. Indeed, mouse Nppa expression was identified in three out of six wildtype mSMGs analysed and human NPPA expression was identified in two out of three biopsies of normal hSMGs. These conserved variations in NPPA expression may result from intrinsic differences between individuals or a timely regulation of NPPA in SGs.
One of the key findings of this investigation is the presence of CNP and its two receptors NPRB and NPRC in autonomic nerves controlling SG function. Widespread CNP expression within nerve bundles strongly suggests CNP is expressed in neural cells and possibly associated glial cells. CNP is a peptide that is abundant in the central nervous system, with a significant amount of CNP detected in the cerebrospinal fluid (Kaneko et al. 1993), while CNP plasma concentration is at the limit of detection (Schulz 2005). It is believed CNP plays an important role in the nervous system as CNP can inhibit proliferation induced by either brain derived neurotrophic factor or nerve growth factor in olfactory neuronal precursors and promote their differentiation (Simpson et al. 2002). In adult mice, CNP has been shown to be expressed by Schwann cells located within dorsal root ganglia and in skeletal muscle nerve bundles (Kishimoto et al. 2008). Impaired neuronal NPRB signalling by overexpression of a dominant-negative NPRB mutant receptor in neurons results in sympathetic excitation, suggesting CNP normally plays a role in dampening sympathetic neurotransmission (Buttgereit et al. 2016; Ajijola et al. 2016). If CNP plays a similar neuromodulator role in the SMG, it is likely to reduce the protein content, and therefore influence the viscosity of saliva. The murine data, showing immunoreactivity for CNP, NprB and NprC in neuronal bodies of mouse submandibular ganglia, yet suggest the NP system may also play a role in the parasympathetic innervation of the SMG. This discovery raises questions about the functional significance of CNP, NprB, NprC and NprA in the peripheral nervous system of the SMG.
NPs could control saliva production via not only the local autonomic innervation but also the glandular microvasculature, a site of exchange of water, nutrients and oxygen. Indeed, CNP, NPRB and NPRC were detected in the blood vessel walls of hSMGs. Furthermore, circulating ANP secreted by the heart is known to bind to receptors in blood vessels, causing vasodilation by relaxing vascular smooth muscles and causing an increase in vascular permeability (Tucker 1996; Renkin and Tucker 1996). Arterioles within human and mouse SMGs express the ANP receptors NPRA and NPRC, enabling them to respond to circulating ANP. Expression of the CNP receptor NPRB in the muscular wall of arterioles of the hSMG supports both endocrine signalling from circulating CNP and paracrine signalling from local CNP found in smooth muscle cells of arterioles. Putative CNP/NPRB signalling appears to be specific to human SG arterioles, since these two natriuretic components are not expressed in murine arteriole walls. While CNP production was identified in endothelial cells of bovine and human carotid artery (Suga et al. 1992b; Stingo et al. 1992), we failed to identify significant levels of CNP in the endothelium of arterioles of salivary glands.
All NP components were identified in ductal cells of SMGs, with the exception of BNP in mSMGs. While similar saliva and plasma levels of ANP indicate little if no secretion of ANP in saliva (Gauquelin et al. 1992), CNP may be secreted in saliva as immunoreactivity for pro-CNP was significantly stronger at the luminal membrane of striated and excretory duct cells in hSMGs. Considering the role of CNP in bone growth (Yasoda et al. 2009), lipid metabolism (Bae et al. 2018b) and its recently reported anti-inflammatory and anti-fibrotic effects (Bae et al. 2018a), it will be important to test the presence of CNP in saliva, which is often used as a diagnostic tool (Javaid et al. 2016). If CNP is secreted in saliva, CNP saliva concentrations could be exploited as an alternative way to assess CNP plasma concentrations, which are known to vary in pathological states, e.g. increased in chronic heart failure (Kalra et al. 2003). It is interesting the three NPs are expressed in human striated ducts, while in the mouse ANP is detected in granular ducts and CNP in striated ducts. Rodent granular and striated ducts as well as human striated ducts play a significant role in modifying saliva composition by actively secreting and reabsorbing substances as primary saliva transits towards the oral cavity. In the kidney, ANP acts on collecting duct cells to inhibit sodium reabsorption through epithelial sodium channels, thereby increasing urinary sodium excretion (Stanton 1991). In normal SGs, especially during low flow periods, granular and striated ducts reabsorb sodium ions rendering the salivary fluid hypotonic. Hypotonic saliva allows taste buds to perceive different tastes that would otherwise be masked by normal plasma sodium levels (Humphrey and Williamson 2001) and allows for hydration and expansion of mucin glycoproteins in the oral cavity, enabling them to protect exposed tissues in the oral cavity (Tabak et al. 1982). ANP administration within the blood circulation does modify the composition of agonist-induced saliva, suggesting circulating ANP, and possibly ANP locally produced in SGs may modulate saliva composition. ANP production in SG ducts may contribute to the regulation of electrolyte reabsorption in an autocrine manner, since NPRA and NPRB, the receptors for ANP, are also expressed in granular and striated ducts. However, the mouse and human data show variable expression of ANP between individuals, which suggests a putative role of ANP in saliva electrolyte regulation is either timely regulated or individual-specific, in which case it could account for differences in taste sensitivity between individuals. BNP and CNP might fulfil similar functions in SG ducts as their receptors, respectively NPRA and NPRC for BNP and NPRB and NPRC for CNP, are present within ductal cells. The presence of some of the components of the natriuretic peptide system in duct cells of other major SGs (Vollmar 1990; Lipari et al. 2013; Cantin et al. 1984), other exocrine glands such as the lacrimal gland (Cho et al. 2000b; Lange et al. 1990) and the salt gland of the Pekin duck (Lange et al. 1989), further strengthens an involvement of this system in the regulation of water and electrolytes in glandular secretions.
In addition to the physiological effects of the NP system, recent research has also identified NPRA expression and signalling in a number of cancers. Overexpression of NPRA correlates with tumour size and disease stage in gastric cancer (Li et al. 2016), with TNM stages and poor prognosis in oesophageal squamous cell carcinomas (Zhao et al. 2014) and the severity of the clinical stage of prostate cancers (Wang et al. 2011). It was demonstrated in the mouse that signalling through NPRA plays a key role in tumorigenesis. Indeed, NprA-deficient mice injected with either lung, skin or ovarian tumour cells showed little tumour growth compared to wild-type control mice (Kong et al. 2008). Although the downstream pathways involved in this anti-tumour effect are not yet known, down-regulation of VEGF in NprA-deficient mice suggests an attenuation of angiogenesis may contribute to the suppression of tumour growth in the absence of NprA. In line with the abnormal overexpression of NPRA in malignant neoplasms, we found NPRA was ectopically expressed in the glandular stroma of SMGs resected from patients diagnosed with OSCC of non-SG origin. Two out of five cases of OSCC presented abnormally high stromal expression of NPRA in resected SMG tissue. Interestingly, ectopic stromal expression of NPRA was observed in another glandular malignancy, namely high-grade prostate cancers (Wang et al. 2011). Considering none of the SMG tissues from healthy patients displayed NPRA stromal expression, it is likely this abnormal finding is linked to the presence of head and neck cancer. These alterations in NPRA immunoreactivity may reflect early molecular changes that have not yet translated into tissue disorganisation, given all of the analysed SMG tissues from head and neck cancer patients appeared histologically normal. Ectopic stromal expression of NPRA may correlate with the stromal invasion of tumours cells (the initial step of metastasis) or the presence of immune cells, some of which being known to express the ANP receptor NPRA (Kiemer and Vollmar 2001; Mohapatra 2007; Tsukagoshi et al. 2001). Alternatively, abnormal stromal NPRA immunoreactivity could reflect non-specific binding of the antibody to a remodelled stroma. Cancer-associated fibroblasts secrete extracellular matrix (ECM) and enzymes that crosslink collagen fibres, thereby stiffening the tissue, a pathological feature that is thought to correlate with an increased risk of metastasis (Egeblad et al. 2010; Kalluri and Zeisberg 2006; Cox et al. 2013). Consistent with a role of NPRA in ECM reorganisation, downregulation of NPRA in human oesophageal SCC suppresses expression of the matrix metalloproteinases MMP2 and MMP9 (Zhao et al. 2014). These data warrant further studies to confirm high stromal NPRA expression at the invasive front of OSCC.
In conclusion, the NP system has the potential to regulate SG homeostasis at three distinct levels, by controlling electrolyte secretion/re-absorption in ducts, modulating the input of the autonomic nervous system and regulating the state of contraction/relaxation of the musculature of small blood vessels. Our exploratory study suggests this system is deregulated in oral malignancies, highlighting the need for further research.
References
Ajijola OA, Shivkumar K, Habecker BA (2016) Natriuretic peptides and peripheral autonomic neurotransmission: back to the A, B, and C's. Cardiovasc Res 112:619–621
Bae CR, Hino J, Hosoda H, Miyazato M, Kangawa K (2018a) C-type natriuretic peptide (CNP) in endothelial cells attenuates hepatic fibrosis and inflammation in non-alcoholic steatohepatitis. Life Sci 209:349–356
Bae CR, Hino J, Hosoda H, Son C, Makino H, Tokudome T, Tomita T, Hosoda K, Miyazato M, Kangawa K (2018b) Adipocyte-specific expression of C-type natriuretic peptide suppresses lipid metabolism and adipocyte hypertrophy in adipose tissues in mice fed high-fat diet. Sci Rep 8:2093
Buttgereit J, Shanks J, Li D, Hao G, Athwal A, Langenickel TH, Wright H, Da Costa Goncalves AC, Monti J, Plehm R, Popova E, Qadri F, Lapidus I, Ryan B, Ozcelik C, Paterson DJ, Bader M, Herring N (2016) C-type natriuretic peptide and natriuretic peptide receptor B signalling inhibits cardiac sympathetic neurotransmission and autonomic function. Cardiovasc Res 112:637–644
Cantin M, Gutkowska J, Thibault G, Milne RW, Ledoux S, Minli S, Chapeau C, Garcia R, Hamet P, Genest J (1984) Immunocytochemical localization of atrial natriuretic factor in the heart and salivary glands. Histochemistry 80:113–127
Cauli A, Yanni G, Panayi GS (1994) Endogenous avidin-binding activity in epithelial cells of the ducts of human salivary glands. Clin Exp Rheumatol 12:45–47
Cho ES, Kim SZ, Cho KW, Park BK (2000a) Immunohistochemical localization of C-type natriuretic peptide in the rat submaxillary salivary gland. Arch Oral Biol 45:425–430
Cho ES, Kim SZ, Kim SH, Park BK, Cho KW (2000b) Natriuretic peptide system in the rat lacrimal gland. Exp Eye Res 71:333–340
Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT (2013) LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 73:1721–1732
Curry FR (2005) Atrial natriuretic peptide: an essential physiological regulator of transvascular fluid, protein transport, and plasma volume. J Clin Invest 115:1458–1461
Dsouza SP, Davis M, Baxter GF (2004) Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther 101:113–129
Egeblad M, Rasch MG, Weaver VM (2010) Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol 22:697–706
Evrard A, Hober C, Racadot A, Lefebvre J, Vantyghem MC (1999) Atrial natriuretic hormone and endocrine functions. Ann Biol Clin (Paris) 57:149–155
Gauquelin G, Maillet A, Allevard AM, Vorobiev D, Grigoriev AI, Gharib C (1992) Presence of atrial natriuretic factor and cyclic guanosine monophosphate in saliva. Comparison of plasma and salivary concentrations during a head-down tilt. Eur J Appl Physiol Occup Physiol 65:25–29
Gutkowska J, Nemer M (1989) Structure, expression, and function of atrial natriuretic factor in extraatrial tissues. Endocr Rev 10:519–536
Hodes A, Lichtstein D (2014) Natriuretic hormones in brain function. Front Endocrinol (Lausanne) 5:201
Houben AJ, Van der Zander K, De Leeuw PW (2005) Vascular and renal actions of brain natriuretic peptide in man: physiology and pharmacology. Fundam Clin Pharmacol 19:411–419
Humphrey SP, Williamson RT (2001) A review of saliva: normal composition, flow, and function. J Prosthet Dent 85:162–169
Javaid MA, Ahmed AS, Durand R, Tran SD (2016) Saliva as a diagnostic tool for oral and systemic diseases. J Oral Biol Craniofac Res 6:66–75
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6:392–401
Kalra PR, Clague JR, Bolger AP, Anker SD, Poole-Wilson PA, Struthers AD, Coats AJ (2003) Myocardial production of C-type natriuretic peptide in chronic heart failure. Circulation 107:571–573
Kaneko T, Shirakami G, Nakao K, Nagata I, Nakagawa O, Hama N, Suga S, Miyamoto S, Kubo H, Hirai O et al (1993) C-type natriuretic peptide (CNP) is the major natriuretic peptide in human cerebrospinal fluid. Brain Res 612:104–109
Kaszuba SM, Zafereo ME, Rosenthal DI, El-Naggar AK, Weber RS (2007) Effect of initial treatment on disease outcome for patients with submandibular gland carcinoma. Arch Otolaryngol Head Neck Surg 133:546–550
Kiemer AK, Vollmar AM (2001) The atrial natriuretic peptide regulates the production of inflammatory mediators in macrophages. Ann Rheum Dis 60(Suppl 3):iii68–iii70
Kishimoto I, Tokudome T, Horio T, Soeki T, Chusho H, Nakao K, Kangawa K (2008) C-type natriuretic peptide is a Schwann cell-derived factor for development and function of sensory neurones. J Neuroendocrinol 20:1213–1223
Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV (1991) Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 252:120–123
Kong X, Wang X, Xu W, Behera S, Hellermann G, Kumar A, Lockey RF, Mohapatra S, Mohapatra SS (2008) Natriuretic peptide receptor a as a novel anticancer target. Cancer Res 68:249–256
Lange W, Unger J, Weindl A, Lang RE (1989) Demonstration of atrial natriuretic peptide/cardiodilatin (ANP/CDD)-immunoreactivity in the salt gland of the Pekin duck. Anat Embryol (Berl) 179:465–469
Lange W, Lang RE, Basting C, Unger JW (1990) Localization of atrial natriuretic peptide/cardiodilatin (ANP/CDD)-immunoreactivity in the lacrimal gland of the domestic pig. Exp Eye Res 50:313–316
Li D, Lu CJ, Hao G, Wright H, Woodward L, Liu K, Vergari E, Surdo NC, Herring N, Zaccolo M, Paterson DJ (2015) Efficacy of B-type natriuretic peptide is coupled to phosphodiesterase 2A in cardiac sympathetic neurons. Hypertension 66:190–198
Li Z, Wang JW, Wang WZ, Zhi XF, Zhang Q, Li BW, Wang LJ, Xie KL, Tao JQ, Tang J, Wei S, Zhu Y, Xu H, Zhang DC, Yang L, Xu ZK (2016) Natriuretic peptide receptor A inhibition suppresses gastric cancer development through reactive oxygen species-mediated G2/M cell cycle arrest and cell death. Free Radic Biol Med 99:593–607
Lipari L, Farina EV, Buscemi M, Gerbino A (2013) Atrial natriuretic peptide presence in parotid gland of human fetus at 13th week of development and in adult man. Folia Histochem Cytobiol 51:55–58
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Mohapatra SS (2007) Role of natriuretic peptide signaling in modulating asthma and inflammation. Can J Physiol Pharmacol 85:754–759
Murthy KS, Teng BQ, Zhou H, Jin JG, Grider JR, Makhlouf GM (2000) G(i–1)/G(i–2)-dependent signaling by single-transmembrane natriuretic peptide clearance receptor. Am J Physiol Gastrointest Liver Physiol 278:G974–G980
Nashida T, Imai A, Shimomura H (1996) Characterization of natriuretic peptide receptors in the rat parotid. Biochem Mol Biol Int 40:111–118
Pagano M, Anand-Srivastava MB (2001) Cytoplasmic domain of natriuretic peptide receptor C constitutes Gi activator sequences that inhibit adenylyl cyclase activity. J Biol Chem 276:22064–22070
Potter LR (2011) Natriuretic peptide metabolism, clearance and degradation. FEBS J 278:1808–1817
Renkin EM, Tucker VL (1996) Atrial natriuretic peptide as a regulator of transvascular fluid balance. News Physiol Sci 11:138–143
Schulz S (2005) C-type natriuretic peptide and guanylyl cyclase B receptor. Peptides 26:1024–1034
Simpson PJ, Miller I, Moon C, Hanlon AL, Liebl DJ, Ronnett GV (2002) Atrial natriuretic peptide type C induces a cell-cycle switch from proliferation to differentiation in brain-derived neurotrophic factor- or nerve growth factor-primed olfactory receptor neurons. J Neurosci 22:5536–5551
Stanton BA (1991) Molecular mechanisms of ANP inhibition of renal sodium transport. Can J Physiol Pharmacol 69:1546–1552
Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC (1992) Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol 263:H1318–H1321
Suda M, Tanaka K, Fukushima M, Natsui K, Yasoda A, Komatsu Y, Ogawa Y, Itoh H, Nakao K (1996) C-type natriuretic peptide as an autocrine/paracrine regulator of osteoblast. Evidence for possible presence of bone natriuretic peptide system. Biochem Biophys Res Commun 223:1–6
Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kambayashi Y, Inouye K et al (1992a) Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 130:229–239
Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H (1992b) Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of "vascular natriuretic peptide system". J Clin Invest 90:1145–1149
Suga S, Itoh H, Komatsu Y, Ogawa Y, Hama N, Yoshimasa T, Nakao K (1993) Cytokine-induced C-type natriuretic peptide (CNP) secretion from vascular endothelial cells–evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology 133:3038–3041
Tabak LA, Levine MJ, Mandel ID, Ellison SA (1982) Role of salivary mucins in the protection of the oral cavity. J Oral Pathol 11:1–17
Tsukagoshi H, Shimizu Y, Kawata T, Hisada T, Shimizu Y, Iwamae S, Ishizuka T, Iizuka K, Dobashi K, Mori M (2001) Atrial natriuretic peptide inhibits tumor necrosis factor-alpha production by interferon-gamma-activated macrophages via suppression of p38 mitogen-activated protein kinase and nuclear factor-kappa B activation. Regul Pept 99:21–29
Tucker VL (1996) Plasma ANP levels and protein extravasation during graded expansion with equilibrated whole blood. Am J Physiol 271:R601–R609
Vollmar AM (1990) Atrial natriuretic peptide in peripheral organs other than the heart. Klin Wochenschr 68:699–708
Vollmar AM, Colbatzky F, Hermanns W, Schulz R (1991) Origin and characterization of atrial natriuretic peptide in the rat parotid gland. Anat Embryol (Berl) 184:331–335
Volpe M, Carnovali M, Mastromarino V (2016) The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci (Lond) 130:57–77
Wang X, Raulji P, Mohapatra SS, Patel R, Hellermann G, Kong X, Vera PL, Meyer-Siegler KL, Coppola D, Mohapatra S (2011) Natriuretic peptide receptor a as a novel target for prostate cancer. Mol Cancer 10:56
Wei CM, Aarhus LL, Miller VM, Burnett JC (1993) Action of C-type natriuretic peptide in isolated canine arteries and veins. Am J Physiol 264:H71–H73
Wu C, Wu F, Pan J, Morser J, Wu Q (2003) Furin-mediated processing of Pro-C-type natriuretic peptide. J Biol Chem 278:25847–25852
Yasoda A, Kitamura H, Fujii T, Kondo E, Murao N, Miura M, Kanamoto N, Komatsu Y, Arai H, Nakao K (2009) Systemic administration of C-type natriuretic peptide as a novel therapeutic strategy for skeletal dysplasias. Endocrinology 150:3138–3144
Zhao Z, Liu H, Yang Y, Sun K, Li M, Zhang J, Cai H, Wang J (2014) Expression of natriuretic peptide receptor-A in esophageal squamous cell carcinomas and the relationship with tumor invasion and migration. World J Surg Oncol 12:154
Acknowledgements
The research was partially financed by LEONPLAST Company SRL di Diego Leone, Palma di Montechiaro, AG Italy (PJ_D03_LeonplastSRL. Rich prot. 23, 13 Janaury 2017).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ahmed, A., Gulino, A., Amayo, S. et al. Natriuretic peptide system expression in murine and human submandibular salivary glands: a study of the spatial localisation of ANB, BNP, CNP and their receptors. J Mol Hist 51, 3–13 (2020). https://doi.org/10.1007/s10735-019-09849-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-019-09849-5