Abstract
Emerging evidence suggest that macrophage and osteoclast are two competing differentiation outcomes from myeloid progenitors. In this review, we summarize recent advances in the understanding of the molecular mechanisms controlling the polarization of macrophage and osteoclast. These include nuclear receptors/transcription factors such as peroxisome proliferator-activated receptor γ (PPARγ) and estrogen-related receptor α (ERRα), their transcription cofactor PPARγ coactivator 1-β (PGC-1β), metabolic factors such as mitochondrial complex I (CI) component NADH:ubiquinone oxidoreductase iron-sulfur protein 4 (Ndufs4), as well as transmembrane receptors such as very-low-density-lipoprotein receptor (VLDLR). These molecular rheostats promote osteoclast differentiation but suppress proinflammatory macrophage activation and inflammation, by acting lineage-intrinsically, systemically or cross generation. These findings provide new insights to the understanding of the interactions between innate immunity and bone remodeling, advancing the field of osteoimmunology.

Similar content being viewed by others
References
Clarke B (2008) Normal bone anatomy and physiology, Clinical. J Am Soc Nephrol 3(Supplement 3):S131–S139
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis*. Endocr Rev 21(2):115–137
Harada S-i, Rodan GA (2003) Control of osteoblast function and regulation of bone mass. Nature 423:349
Seeman E, Delmas PD (2006) Bone quality — the material and structural basis of bone strength and fragility. N Engl J Med 354(21):2250–2261
Aubin JE (2001) Regulation of osteoblast formation and function. Rev Endocr Metab Disord 2(1):81–94
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289(5484):1504–1508
Zelzer E, Olsen BR (2003) The genetic basis for skeletal diseases. Nature 423:343
Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7:292
Nakashima T, Hayashi M, Takayanagi H (2012) New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab 23(11):582–590
Tacken PJ, Teusink B, Jong MC, Harats D, Havekes LM, van Dijk KW, Hofker MH (2000) LDL receptor deficiency unmasks altered VLDL triglyceride metabolism in VLDL receptor transgenic and knockout mice. J Lipid Res 41(12):2055–2062
Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638
Theill LE, Boyle WJ, Penninger JM (2002) RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 20(1):795–823
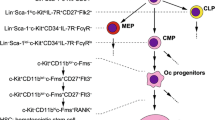
Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER, Kurokawa T, Suda T (1993) Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest 91(1):257–263
Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T (1999) Commitment and differentiation of osteoclast precursor cells by the sequential expression of C-Fms and receptor activator of nuclear factor κb (Rank) receptors. J Exp Med 190(12):1741–1754
Ross FP, Teitelbaum SL (2005) αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev 208(1):88–105
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175
Fuller K, Wong B, Fox S, Choi Y, Chambers TJ (1998) TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med 188(5):997–1001
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93(2):165–176
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S-i, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci 95(7):3597–3602
Wong BR, Josien R, Choi Y (1999) TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. J Leukoc Biol 65(6):715–724
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89(2):309–319
Tsuda E, Goto M, Mochizuki S-i, Yano K, Kobayashi F, Morinaga T, Higashio K (1997) Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun 234(1):137–142
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17:1235
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231
Xiong J, Piemontese M, Thostenson JD, Weinstein RS, Manolagas SC, O'Brien CA (2014) Osteocyte-derived RANKL is a critical mediator of the increased bone resorption caused by dietary calcium deficiency. Bone 66:146–154
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J-i, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3(6):889–901
Arron JR, Choi Y (2000) Bone versus immune system. Nature 408:535
Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T (2000) T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408:600
Kim N, Odgren PR, Kim DK, Marks SC Jr, Choi Y (2000) Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci U S A 97(20):10905–10910
Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y (2006) Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 24(1):33–63
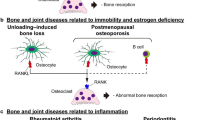
S.R. Goldring, Pathogenesis of bone and cartilage destruction in rheumatoid arthritis, Rheumatology 42(suppl_2) (2003) ii11-ii16
Walsh NC, Gravallese EM (2004) Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr Opin Rheumatol 16(4):419–427
Lorenzo J, Horowitz M, Choi Y (2008) Osteoimmunology: interactions of the bone and immune system. Endocr Rev 29(4):403–440
Varol C, Mildner A, Jung S (2015) Macrophages: Development and tissue specialization. Annu Rev Immunol 33(1):643–675
Chang MK, Raggatt L-J, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR (2008) Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 181(2):1232–1244
Sinder BP, Pettit AR, McCauley LK (2015) Macrophages: their emerging roles in bone. J Bone Miner Res 30(12):2140–2149
Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM, Cope AP, Horwood NJ (2012) Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS One 7(7):e39871
Athanasou NA, Heryet A, Quinn J, Gatter KC, Mason DY, McGe JOD (1986) Osteoclasts contain macrophage and megakaryocyte antigens. J Pathol 150(4):239–246
Athanasou NA, Quinn J (1990) Immunophenotypic differences between osteoclasts and macrophage polykaryons: immunohistological distinction and implications for osteoclast ontogeny and function. J Clin Pathol 43(12):997–1003
Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405:421
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (1995) The nuclear receptor superfamily: the second decade. Cell 83(6):835–839
T. Lemberger, BD and, W. Wahli, Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology, Annu Rev Cell Dev Biol 12(1) (1996) 335–363
Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J Biol Chem 270(22):12953–12956
Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM (1995) A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 83(5):813–819
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM (1999) PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4(4):611–617
Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK (1998) The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391:79
Rangwala SM, Lazar MA (2004) Peroxisome proliferator-activated receptor γ in diabetes and metabolism. Trends Pharmacol Sci 25(6):331–336
Moore KJ, Rosen ED, Fitzgerald ML, Randow F, Andersson LP, Altshuler D, Milstone DS, Mortensen RM, Spiegelman BM, Freeman MW (2001) The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nat Med 7:41
Tontonoz P, Nagy L, Alvarez JGA, Thomazy VA, Evans RM (1998) PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93(2):241–252
Heming M, Gran S, Jauch S-L, Fischer-Riepe L, Russo A, Klotz L, Hermann S, Schäfers M, Roth J, Barczyk-Kahlert K (2018) Peroxisome proliferator-activated receptor-γ modulates the response of macrophages to lipopolysaccharide and glucocorticoids. Front Immunol 9(893)
Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM (2001) PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med 7:48
Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M (2014) Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol 15:1026
Jiang C, Ting AT, Seed B (1998) PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82
Wan Y, Evans RM (2010) Rosiglitazone activation of PPARγ suppresses fractalkine signaling. J Mol Endocrinol 44(2):135–142
Wu L, Yan C, Czader M, Foreman O, Blum JS, Kapur R, Du H (2012) Inhibition of PPARγ in myeloid-lineage cells induces systemic inflammation, immunosuppression, and tumorigenesis. Blood 119(1):115–126
Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A (2007) Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447:1116
Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G (2007) PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6(2):137–143
Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart J-C, Chapman J, Najib J, Staels B (1998) Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. J Biol Chem 273(40):25573–25580
Bodles AM, Varma V, Yao-Borengasser A, Phanavanh B, Peterson CA, McGehee RE, Rasouli N, Wabitsch M, Kern PA (2006) Pioglitazone induces apoptosis of macrophages in human adipose tissue. J Lipid Res 47(9):2080–2088
Saltiel AR, Olefsky JM (1996) Thiazolidinediones in the treatment of insulin resistance and type ii diabetes. Diabetes 45(12):1661–1669
Soccio RE, Chen ER, Lazar MA (2014) Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab 20(4):573–591
Grey A, Horne A, Wattie D, Reid IR, Bolland M, Gamble G, Davidson J (2007) The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 92(4):1305–1310
Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355(23):2427–2443
Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu DH, Heise MA, Aftring RP, Viberti G, for the A Diabetes Outcome Progression Trial (ADOPT) study group (2008) Rosiglitazone-Associated Fractures in Type 2 Diabetes An analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care 31(5):845–851
Rzonca SO, Lecka-Czernik B, Gaddy D, Montague DC, Suva LJ (2004) Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145(1):401–406
Sottile V, Seuwen K, Kneissel M (2004) Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone). Calcif Tissue Int 75(4):329–337
Wan Y, Chong L-W, Evans RM (2007) PPAR-γ regulates osteoclastogenesis in mice. Nat Med 13:1496
Grigoriadis A, Wang Z, Cecchini M, Hofstetter W, Felix R, Fleisch H, Wagner E (1994) c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266(5184):443–448
Wei W, Zeve D, Wang X, Du Y, Tang W, Dechow PC, Graff JM, Wan Y (2011) Osteoclast progenitors reside in the peroxisome proliferator-activated receptor γ-expressing bone marrow cell population. Mol Cell Biol 31(23):4692–4705
Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung U-i, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H (2004) PPAR γ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113(6):846–855
Lazarenko OP, Rzonca SO, Lecka-Czernik B, Swain FL, Suva LJ, Hogue WR (2007) Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology 148(6):2669–2680
Wan Y (2010) PPARγ in bone homeostasis. Trends Endocrinol Metab 21(12):722–728
Stechschulte LA, Czernik PJ, Rotter ZC, Tausif FN, Corzo CA, Marciano DP, Asteian A, Zheng J, Bruning JB, Kamenecka TM, Rosen CJ, Griffin PR, Lecka-Czernik B (2016) PPARG post-translational modifications regulate bone formation and bone resorption. EBioMedicine 10:174–184
Choi S, Chung S, Park K (2018) Re-highlighting the action of PPAR? in treating metabolic diseases [version 1; referees: 2 approved]. F1000Research 7(1127)
Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y (2010) PGC1β mediates pparγ activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab 12(2):202
Wei W, Schwaid AG, Wang X, Wang X, Chen S, Chu Q, Saghatelian A, Wan Y (2016) Ligand activation of ERRα by cholesterol mediates statin and bisphosphonate effects. Cell Metab 23(3):479–491
Giguère V, Yang N, Segui P, Evans RM (1988) Identification of a new class of steroid hormone receptors. Nature 331(6151):91–94
Hong H, Yang L, Stallcup MR (1999) Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem 274(32):22618–22626
Heard DJ, Vissing H, Norby PL, Holloway J (2000) Human ERRγ, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol 14(3):382–392
Giguére V (2002) To ERR in the estrogen pathway. Trends Endocrinol Metab 13(5):220–225
Kallen J, Schlaeppi J-M, Bitsch F, Filipuzzi I, Schilb A, Riou V, Graham A, Strauss A, Geiser M, Fournier B (2004) Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor α (ERRα): crystal structure of ERRα ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1α. J Biol Chem 279(47):49330–49337
Bonnelye E, Vanacker JM, Spruyt N, Alric S, Fournier B, Desbiens X, Laudet V (1997) Expression of the estrogen-related receptor 1 (ERR-1) orphan receptor during mouse development. Mech Dev 65(1):71–85
Giguère V (2008) Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev 29(6):677–696
Audet-walsh É, Giguére V (2014) The multiple universes of estrogen-related receptor α and γ in metabolic control and related diseases. Acta Pharmacol Sin 36:51
Luo J, Sladek R, Carrier J, Bader J-A, Richard D, Giguère V (2003) Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor α. Mol Cell Biol 23(22):7947–7956
Huss JM, Imahashi K-i, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguère V, Murphy E, Kelly DP (2007) The nuclear receptor ERRα is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab 6(1):25–37
Vanacker JM, Pettersson K, Gustafsson JÅ, Laudet V (1999) Transcriptional targets shared by estrogen receptor-related receptors (ERRs) and estrogen receptor (ER) α, but not by ERβ. EMBO J 18(15):4270–4279
Sladek R, Bader JA, Giguère V (1997) The orphan nuclear receptor estrogen-related receptor alpha is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol 17(9):5400–5409
Vega R, Kelly D (1997) A role for estrogen-related receptor α in the control of mitochondrial fatty acid β-oxidation during brown adipocyte differentiation. J Biol Chem 272:31693–31699
Huss JM, Kopp RP, Kelly DP (2002) Peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ: identification of novel leucine-rich interaction motif within PGC-1α. J Biol Chem 277(43):40265–40274
Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A (2003) PPARγ coactivator 1β/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci 100(21):12378–12383
Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A (2003) The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor α (ERRα). J Biol Chem 278(11):9013–9018
Wende AR, Huss JM, Schaeffer PJ, Giguère V, Kelly DP (2005) PGC-1α coactivates PDK4 gene expression via the orphan nuclear receptor ERRα: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol 25(24):10684–10694
Sonoda J, Laganière J, Mehl IR, Barish GD, Chong L-W, Li X, Scheffler IE, Mock DC, Bataille AR, Robert F (2007) Nuclear receptor ERRα and coactivator PGC-1β are effectors of IFN-γ-induced host defense. Genes Dev 21(15):1909–1920
Yuk J-M, Kim TS, Kim SY, Lee H-M, Han J, Dufour CR, Kim JK, Jin HS, Yang C-S, Park K-S, Lee C-H, Kim J-M, Kweon GR, Choi H-S, Vanacker J-M, Moore DD, Giguère V, Jo E-K (2015) Orphan nuclear receptor ERRα controls macrophage metabolic signaling and A20 expression to negatively regulate TLR-induced inflammation. Immunity 43(1):80–91
He X, Ma S, Tian Y, Wei C, Zhu Y, Li F, Zhang P, Wang P, Zhang Y, Zhong H (2017) ERRα negatively regulates type I interferon induction by inhibiting TBK1-IRF3 interaction. PLoS Pathog 13(6):e1006347
Kim SY, Yang C-S, Lee H-M, Kim JK, Kim Y-S, Kim Y-R, Kim J-S, Kim TS, Yuk J-M, Dufour CR, Lee S-H, Kim J-M, Choi H-S, Giguère V, Jo E-K (2018) ESRRA (estrogen-related receptor α) is a key coordinator of transcriptional and post-translational activation of autophagy to promote innate host defense. Autophagy 14(1):152–168
Bae S, Lee MJ, Mun SH, Giannopoulou EG, Yong-Gonzalez V, Cross JR, Murata K, Giguère V, van der Meulen M, Park-Min K-H (2017) MYC-dependent oxidative metabolism regulates osteoclastogenesis via nuclear receptor ERRα. J Clin Invest 127(7):2555–2568
Ocampo CB, Downes M, Yu RT, Evans RM, Barish GD, Alaynick WA, Bookout AL, Mangelsdorf DJ (2005) A nuclear receptor atlas: macrophage activation. Mol Endocrinol 19(10):2466–2477
Bonnelye E, Kung V, Aubin JE, Laplace C, Galson DL (2002) Estrogen receptor-related receptor α impinges on the estrogen axis in bone: potential function in osteoporosis. Endocrinology 143(9):3658–3670
Bonnelye E, Saltel F, Chabadel A, Zirngibl RA, Aubin JE, Jurdic P (2010) Involvement of the orphan nuclear estrogen receptor-related receptor α in osteoclast adhesion and transmigration. J Mol Endocrinol 45(6):365–377
Studer A, Morvan F, Evans G, Delhon I, Wyder L, Kneissel M, Gutzwiller S, Fournier B, Rangwala S (2009) Absence of estrogen receptor-related-α increases osteoblastic differentiation and cancellous bone mineral density. Endocrinology 150(10):4463–4472
Teyssier C, Gallet M, Rabier B, Monfoulet L, Dine J, Macari C, Espallergues J, Horard B, Giguère V, Cohen-Solal M, Chassande O, Vanacker J-M (2009) Absence of ERRα in female mice confers resistance to bone loss induced by age or estrogen-deficiency. PLoS One 4(11):e7942
Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM (2002) Peroxisome proliferator-activated receptor γ coactivator 1β (PGC-1β), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem 277(3):1645–1648
Lin J, Tarr PT, Yang R, Rhee J, Puigserver P, Newgard CB, Spiegelman BM (2003) PGC-1β in the regulation of hepatic glucose and energy metabolism. J Biol Chem 278(33):30843–30848
St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM (2003) Bioenergetic analysis of peroxisome proliferator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J Biol Chem 278(29):26597–26603
Sonoda J, Mehl IR, Chong L-W, Nofsinger RR, Evans RM (2007) PGC-1β controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci 104(12):5223–5228
Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, Kelly DP (2008) Transcriptional coactivators PGC-1α and PGC-lβ control overlapping programs required for perinatal maturation of the heart. Genes Dev 22(14):1948–1961
Vianna CR, Huntgeburth M, Coppari R, Choi CS, Lin J, Krauss S, Barbatelli G, Tzameli I, Kim Y-B, Cinti S, Shulman GI, Spiegelman BM, Lowell BB (2006) Hypomorphic mutation of PGC-1β causes mitochondrial dysfunction and liver insulin resistance. Cell Metab 4(6):453–464
Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly-Y M, Storlien L, Strömstedt M, Snaith M, Orešič M, Abel ED, Cannon B, Vidal-Puig A (2006) Ablation of PGC-1β results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4(11):e369
Gali Ramamoorthy T, Laverny G, Schlagowski A-I, Zoll J, Messaddeq N, Bornert J-M, Panza S, Ferry A, Geny B, Metzger D (2015) The transcriptional coregulator PGC-1β controls mitochondrial function and anti-oxidant defence in skeletal muscles. Nat Commun 6:10210
Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM (2007) The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5(1):35–46
Bellafante E, Murzilli S, Salvatore L, Latorre D, Villani G, Moschetta A (2013) Hepatic-specific activation of peroxisome proliferator-activated receptor γ coactivator-1β protects against steatohepatitis. Hepatology 57(4):1343–1356
Enguix N, Pardo R, González A, López VM, Simó R, Kralli A, Villena JA (2013) Mice lacking PGC-1β in adipose tissues reveal a dissociation between mitochondrial dysfunction and insulin resistance. Mol Metab 2(3):215–226
Chambers KT, Chen Z, Crawford PA, Fu X, Burgess SC, Lai L, Leone TC, Kelly DP, Finck BN (2012) Liver-specific PGC-1beta deficiency leads to impaired mitochondrial function and lipogenic response to fasting-refeeding. PLoS One 7(12):e52645
Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Greaves DR, Murray PJ, Chawla A (2006) Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab 4(1):13–24
Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen H-J, Boshuizen MCS, Ahmed M, Hoeksema MA, de Vos AF, de Winther MPJ (2016) Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep 17(3):684–696
Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, Horng T (2013) The TSC-mTOR pathway regulates macrophage polarization. Nat Commun 4:2834
Chen H, Liu Y, Li D, Song J, Xia M (2016) PGC-1β suppresses saturated fatty acid-induced macrophage inflammation by inhibiting TAK1 activation. IUBMB Life 68(2):145–155
Eisele PS, Furrer R, Beer M, Handschin C (2015) The PGC-1 coactivators promote an anti-inflammatory environment in skeletal muscle in vivo. Biochem Biophys Res Commun 464(3):692–697
Ishii K-a, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ, Vidal-Puig A, Ikeda K (2009) Coordination of PGC-1β and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15:259
Carroll J, Fearnley IM, Shannon RJ, Hirst J, Walker JE (2003) Analysis of the subunit composition of complex I from bovine heart mitochondria. Mol Cell Proteomics 2(2):117–126
Carroll J, Fearnley IM, Skehel JM, Shannon RJ, Hirst J, Walker JE (2006) Bovine complex I is a complex of 45 different subunits. J Biol Chem 281(43):32724–32727
D.M. Kirby, M. Crawford, M.A. Cleary, H.-H.M. Dahl, X. Dennett, D.R. Thorburn, Respiratory chain complex I deficiency: an underdiagnosed energy generation disorder 52(6) (1999) 1255–1255
Mimaki M, Wang X, McKenzie M, Thorburn DR, Ryan MT (2012) Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta, Bioenerg 1817(6):851–862
Finsterer J (2006) Central nervous system manifestations of mitochondrial disorders. Acta Neurol Scand 114(4):217–238
Coskun P, Wyrembak J, Schriner SE, Chen H-W, Marciniack C, LaFerla F, Wallace DC (2012) A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim Biophys Acta Gen Subj 1820(5):553–564
Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD (2008) Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab 7(4):312–320
Scacco S, Petruzzella V, Budde S, Vergari R, Tamborra R, Panelli D, van den Heuvel LP, Smeitink JA, Papa S (2003) Pathological mutations of the human NDUFS4 gene of the 18-kDa (AQDQ) subunit of complex I affect the expression of the protein and the assembly and function of the complex. J Biol Chem 278(45):44161–44167
Petruzzella V, Papa S (2002) Mutations in human nuclear genes encoding for subunits of mitochondrial respiratory complex I: the NDUFS4 gene. Gene 286(1):149–154
Budde SMS, van den Heuvel LPWJ, Smeets RJP, Skladal D, Mayr JA, Boelen C, Petruzzella V, Papa S, Smeitink JAM (2003) Clinical heterogeneity in patients with mutations in the NDUFS4 gene of mitochondrial complex I. J Inherit Metab Dis 26(8):813–815
Ugalde C, Smeitink JAM, van den Heuvel LP, Nijtmans LGJ, Janssen RJRJ (2004) Differences in assembly or stability of complex I and other mitochondrial OXPHOS complexes in inherited complex I deficiency. Hum Mol Genet 13(6):659–667
van den Heuvel L, Ruitenbeek W, Smeets R, Gelman-Kohan Z, Elpeleg O, Loeffen J, Trijbels F, Mariman E, de Bruijn D, Smeitink J (1998) Demonstration of a new pathogenic mutation in human complex I deficiency: a 5-bp duplication in the nuclear gene encoding the 18-kD (AQDQ) subunit. Am J Hum Genet 62(2):262–268
Iuso A, Scacco S, Piccoli C, Bellomo F, Petruzzella V, Trentadue R, Minuto M, Ripoli M, Capitanio N, Zeviani M, Papa S (2006) Dysfunctions of cellular oxidative metabolism in patients with mutations in the NDUFS1 and NDUFS4 genes of complex I. J Biol Chem 281(15):10374–10380
Quintana A, Kruse SE, Kapur RP, Sanz E, Palmiter RD (2010) Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc Natl Acad Sci 107(24):10996–11001
Jin Z, Wei W, Yang M, Du Y, Wan Y (2014) Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab 20(3):483–498
Oka K, Ishimura-Oka K, Chu M-j, Sullivan M, Krushkal J, Li W-H, Chan L (1994) Mouse very-low-density-lipoprotein receptor (VLDLR) cDNA cloning, tissue-specific expression and evolutionary relationship with the low-density-lipoprotein receptor. Eur J Biochem 224(3):975–982
Webb JC, Patel DD, Jones MD, Knight BL, Soutar AK (1994) Characterization and tissue-specific expression of the human ‘very low density lipoprotein (VLDL) receptor’mRNA. Hum Mol Genet 3(4):531–537
Tacken PJ, Hofker MH, Havekes LM, van Dijk KW (2001) Living up to a name: the role of the VLDL receptor in lipid metabolism. Curr Opin Lipidol 12(3):275–279
Takahashi S, Sakai J, Fujino T, Hattori H, Zenimaru Y, Suzuki J, Miyamori I, Yamamoto TT (2004) The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J Atheroscler Thromb 11(4):200–208
Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J (1999) Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97(6):689–701
D'Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T (1999) Reelin is a ligand for lipoprotein receptors. Neuron 24(2):471–479
Goudriaan JR, Tacken PJ, Dahlmans VE, Gijbels MJ, van Dijk KW, Havekes LM, Jong MC (2001) Protection from obesity in mice lacking the VLDL receptor. Arterioscler Thromb Vasc Biol 21(9):1488–1493
Nguyen A, Tao H, Metrione M, Hajri T (2014) Very low density lipoprotein receptor (VLDLR) expression is a determinant factor in adipose tissue inflammation and adipocyte-macrophage interaction. J Biol Chem 289(3):1688–1703
Eck MV, Oost J, Goudriaan JR, Hoekstra M, Hildebrand RB, Bos IST, van Dijk KW, Van Berkel TJC (2005) Role of the macrophage very-low-density lipoprotein receptor in atherosclerotic lesion development. Atherosclerosis 183(2):230–237
Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J (1995) Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc Natl Acad Sci 92(18):8453–8457
Miyamoto T, Arai F, Ohneda O, Takagi K, Anderson DM, Suda T (2000) An adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor κB ligand. Blood 96(13):4335–4343
Du Y, Yang M, Wei W, Huynh HD, Herz J, Saghatelian A, Wan Y (2012) Macrophage VLDL receptor promotes PAFAH secretion in mother’s milk and suppresses systemic inflammation in nursing neonates. Nat Commun 3:1008–1008
Huynh H, Wei W, Wan Y (2017) mTOR inhibition subdues milk disorder caused by maternal VLDLR loss. Cell Rep 19(10):2014–2025
Yang D, Huynh H, Wan Y (2018) Milk lipid regulation at the maternal-offspring interface. Semin Cell Dev Biol 81:141–148
Caplan MS, Sun X-M, Hsueh W, Hageman JR (1990) Role of platelet activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis. J Pediatr 116(6):960–964
Walker A (2010) Breast milk as the gold standard for protective nutrients. J Pediatr 156(2, Supplement):S3–S7
Furukawa M, Narahara H, Yasuda K, Johnston JM (1993) Presence of platelet-activating factor-acetylhydrolase in milk. J Lipid Res 34(9):1603–1609
Moya FR, Eguchi H, Zhao B, Furukawa M, Sfeir J, Osorio M, Ogawa Y, Johnston JM (1994) Platelet-activating factor acetylhydrolase in term and preterm human milk: a preliminary report. J Pediatr Gastroenterol Nutr 19(2):236–239
Shin KC, Hwang I, Choe SS, Park J, Ji Y, Kim JI, Lee GY, Choi SH, Ching J, Kovalik J-P, Kim JB (2017) Macrophage VLDLR mediates obesity-induced insulin resistance with adipose tissue inflammation. Nat Commun 8(1):1087
Huang SC-C, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, Pearce EJ (2016) Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity 45(4):817–830
Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Däbritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, O’Neill LA (2016) Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167(2):457–470.e13
Acknowledgments
Y.W. is Lawrence Raisz Professor in Bone Cell Metabolism and a Virginia Murchison Linthicum Scholar in Medical Research.
Funding
This work was in part supported by NIH (R01CA229487, R01CA236802, Y.W.), DOD (W81XWH-18-1-0014, Y.W.), CPRIT (RP180047, Y.W.), The Welch Foundation (I-1751, Y.W.), and UT Southwestern Endowed Scholar Startup Fund (Y.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is a contribution to the special issue on Osteoimmunology - Guest Editor: Mary Nakamura
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, D., Wan, Y. Molecular determinants for the polarization of macrophage and osteoclast. Semin Immunopathol 41, 551–563 (2019). https://doi.org/10.1007/s00281-019-00754-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-019-00754-3