Abstract
Purpose
The present study was aimed to demonstrate the recuperative effect of nesfatin-1 on testicular dysfunction in the high-fat diet (HFD)/streptozotocin (STZ)-induced type-2 diabetes mellitus (T2DM) mice.
Method and results
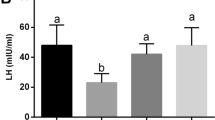
Three experimental groups were formed: (1) vehicle control (VC), (2) T2DM mice, (3) T2DM + nesf-1. The mice with blood glucose level higher than 300 mg/dL following HFD and a single dose of STZ were used for the experiment. The T2DM mice showed increases in body mass, blood glucose and insulin levels, reductions in spermatogenesis and steroidogenesis, production of antioxidative enzymes, and disturbed lipid profile. These alterations were all ameliorated by administration of nesfatin-1 at 20 μg/Kg BW for 15 days. Nesfatin-1 treatment also increased the production of testosterone (T), improved insulin sensitivity, and effectively ameliorated the testicular aberrations, and increased spermatogenesis and steroidogenesis. In addition, nesfatin-1 treatment upregulated the PCNA and Bcl2 expression and inhibited the caspase-3 and prohibitin expression in T2DM mice. Nesfatin-1 increased insulin receptor (IR) and GLUT8 expressions, and lactate production, the changes that further substantiate the increase of energy influx to the testis.
Conclusion
Altogether, the results suggest the ameliorative effect of nesfatin-1 against T2DM-associated testicular dysfunctions and improved insulin sensitivity along with promoting T production and fertility in T2DM mice.





Similar content being viewed by others
References
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48(1):1–9. https://doi.org/10.2337/diabetes.48.1.1
Yu D, Zheng W, Cai H et al (2018) Long-term diet quality and risk of type 2 diabetes among urban Chinese adults. Diabetes Care 41(4):723–730. https://doi.org/10.2337/dc17-1626
Nah WH, Koh IK, Ahn HS et al (2012) Effect of Spirulina maxima on spermatogenesis and steroidogenesis in streptozotocin-induced type-I diabetic male rats. Food Chem 134(1):173–179. https://doi.org/10.1109/PHOSST.2012.6280702
Maresch CC, Stute DC, Alves MG et al (2018) Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review. Hum Reprod Update 24(1):86–105. https://doi.org/10.1093/humupd/dmx033
Long L, Qiu H, Cai B et al (2018) Hyperglycemia induced testicular damage in type 2 diabetes mellitus rats exhibiting microcirculation impairments associated with vascular endothelial growth factor decreased via PI3K/Akt pathway. Oncotarget 9(4):5321–5336. https://doi.org/10.18632/oncotarget.23915
Al-Kuraishy HM, Al-Gareeb AI (2016) Erectile dysfunction and low sex drive in men with type 2 DM: the potential role of diabetic pharmacotherapy. J Clin Diagnostic Res 10(12):FC21–FC26. https://doi.org/10.7860/jcdr/2016/19971.8996
Grossmann M, Thomas MC, Panagiotopoulos S et al (2008) Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 93(5):1834–1840. https://doi.org/10.1210/jc.2007-2177
Dong J, Xu H, Wang PF et al (2013) Nesfatin-1 stimulates fatty-acid oxidation by activating AMP-activated protein kinase in STZ-induced type 2 diabetic mice. PLoS One 8(12):1–8. https://doi.org/10.1371/journal.pone.0083397
Ranjan A, Choubey M, Yada T, Krishna A (2018) Direct effects of neuropeptide nesfatin-1 on testicular spermatogenesis and steroidogenesis of the adult mice. Gen Comp Endocrinol 271:49–60. https://doi.org/10.1016/j.ygcen.2018.10.022
Gao X, Zhang K, Song M et al (2016) Role of nesfatin-1 in the reproductive axis of male rat. Sci Rep 6(130):1–10. https://doi.org/10.1038/srep32877
Oh-I S, Shimizu H, Satoh T et al (2006) Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443(7112):709–712. https://doi.org/10.1038/nature05162
Brailoiu GC, Dun SL, Brailoiu E et al (2007) Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 148(10):5088–5094. https://doi.org/10.1210/en.2007-0701
Shimizu H, Oh-I S, Okada S, Mori M (2009) Nesfatin-1: an overview and future clinical application. Endocr J 56(4):537–543. https://doi.org/10.1507/endocrj.K09E-117
Goebel M, Stengel A, Wang L, Taché Y (2011) Central nesfatin-1 reduces the nocturnal food intake in mice by reducing meal size and increasing inter-meal intervals. Peptides 32(1):36–43. https://doi.org/10.1016/j.peptides.2010.09.027
Kim J, Yang H (2012) Nesfatin-1 as a new potent regulator in reproductive system jinhee. Dev Reprod 16(4):253–264
García-Galiano D, Pineda R, Ilhan T et al (2012) Cellular distribution, regulated expression, and functional role of the anorexigenic peptide, NUCB2/nesfatin-1, in the testis. Endocrinology 153(4):1959–1971. https://doi.org/10.1210/en.2011-2032
Kohno D, Nakata M, Maejima Y et al (2008) Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology 149(3):1295–1301. https://doi.org/10.1210/en.2007-1276
Maejima Y, Sedbazar U, Suyama S et al (2009) Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 10(5):355–365. https://doi.org/10.1016/j.cmet.2009.09.002
Li QC, Wang HY, Chen X, Guan HZ, Jiang ZY (2010) Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul Pept 159(1–3):72–77. https://doi.org/10.1016/j.regpep.2009.11.003
Riva M, Nitert MD, Voss U et al (2011) Nesfatin-1 stimulates glucagon and insulin secretion and beta cell NUCB2 is reduced in human type 2 diabetic subjects. Cell Tissue Res 346(3):393–405. https://doi.org/10.1007/s00441-011-1268-5
Guler S, Simsek Y, Kocabas R, Gorkem U, Gulen S, Kucukler FK (2016) Low level of nesfatin-1 is associated with gestational diabetes mellitus. Gynecol Endocrinol 32(9):759–761. https://doi.org/10.1080/09513590.2016.1180679
Su Y, Zhang J, Tang Y, Bi F, Liu J (2010) Biochemical and biophysical research communications the novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun 391(1):1039–1042. https://doi.org/10.1016/j.bbrc.2009.12.014
Dore R, Levata L, Lehnert H, Schulz C (2017) Nesfatin-1: functions and physiology of a novel regulatory peptide. J Endocrinol 232(1):R45–R65. https://doi.org/10.1530/JOE-16-0361
Nakata M, Manaka K, Yamamoto S et al (2011) Nesfatin-1 enhances glucose-induced insulin secretion by promoting Ca2+ influx through l-type channels in mouse islet β-cells. Endocr J 58(4):305–313. https://doi.org/10.1507/endocrj.K11E-056
Garcia-Galiano D, Navarro VM, Roa J et al (2010) The anorexigenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J Neurosci 30(23):7783–7792. https://doi.org/10.1523/JNEUROSCI.5828-09.2010
Jiang G, Wang M, Wang L et al (2015) The protective effect of nesfatin-1 against renal ischemia-reperfusion injury in rats. Ren Fail 37(5):882–889. https://doi.org/10.3109/0886022X.2015.1015426
Verma R, Samanta R, Krishna A (2018) Comparative effects of estrogen and phytoestrogen, genistein on testicular activities of streptozotocin-induced type 2 diabetic mice. Reprod Sci. https://doi.org/10.1177/1933719118815576
Ahn SW, Gang GT, Kim YD et al (2013) Insulin directly regulates steroidogenesis via induction of the orphan nuclear receptor DAX-1 in testicular Leydig cells. J Biol Chem 288(22):15937–15946. https://doi.org/10.1074/jbc.M113.451773
van Dijk TH, Laskewitz AJ, Grefhorst A et al (2013) A novel approach to monitor glucose metabolism using stable isotopically labelled glucose in longitudinal studies in mice. Lab Anim 47(2):79–88. https://doi.org/10.1177/0023677212473714
Russell LD, Ettlin RA, Hikim APS, Clegg ED (1990) Histological and histopathological evaluation of the testis. Cache River Press, Clearwater, pp 210–264
Choubey M, Ranjan A, Bora PS, Baltazar F, Martin LJ, Krishna A (2019) Role of adiponectin as a modulator of testicular function during aging in mice. Biochim Biophys Acta - Mol Basis Dis 1865(2):413–427. https://doi.org/10.1016/j.bbadis.2018.11.019
Banerjee A, Anuradha Mukherjee K, Krishna A (2014) Testicular glucose and its transporter GLUT 8 as a marker of age-dependent variation and its role in steroidogenesis in mice. J Exp Zool Part A Ecol Genet Physiol 321(9):490–502. https://doi.org/10.1002/jez.1881
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys 37(3):201–204
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–684
Mantha V, Prasad M, Kalra J, Prasad K (1993) Subrahmanyam. Antioxidant enzymes in hypercholesterolemia and effects of vitamin E in rabbits. Atherosclerosis 101:135–144
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide - Biol Chem 5(1):62–71. https://doi.org/10.1006/niox.2000.0319
Eckel RH, Kahn SE, Ferrannini E et al (2011) Obesity and type 2 diabetes: What Can be unified and what needs to be individualized? Diabetes Care 34(6):1424–1430. https://doi.org/10.2337/dc11-0447
Dhindsa S, Ghanim H, Batra M, Dandona P (2018) Hypogonadotropic hypogonadism in men with diabesity. Diabetes Care 41(7):1516–1525. https://doi.org/10.2337/dc17-2510
Hoffler U, Hobbie K, Wilson R et al (2009) Diet-induced obesity is associated with hyperleptinemia, hyperinsulinemia, hepatic steatosis, and glomerulopathy in C57Bl/6J mice. Endocrine 36(2):311–325. https://doi.org/10.1007/s12020-009-9224-9
Contreras PH, Serrano FG, Salgado AM, Vigil P (2018) Insulin sensitivity and testicular function in a cohort of adult males suspected of being insulin-resistant. Front Med 5:1–9. https://doi.org/10.3389/fmed.2018.00190
Dandona P, Dhindsa S (2011) Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab 96(9):2643–2651. https://doi.org/10.1210/jc.2010-2724
Jangir R, Jain G (2014) Diabetes mellitus induced impairment of male reproductive functions: a review. Curr Diabetes Rev 10(3):147–157. https://doi.org/10.2174/1573399810666140606111745
So Temidayo, Stefan SP (2017) Diabetes mellitus and male infertility. Asian Pacific J Reprod 7(1):6. https://doi.org/10.4103/2305-0500.220978
Kovacs P, Parlow AF, Karkanias GB (2002) Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology 76(6):357–365. https://doi.org/10.1159/000067585
Kim ST, Moley KH (2007) The expression of GLUT8, GLUT9a, and GLUT9b in the mouse testis and sperm. Reprod Sci 14(5):445–455
Chen Y, Nagpal ML, Lin T (2003) Expression and regulation of glucose transporter 8 in rat Leydig cells. J Endocrinol 179:63–72
Alves MG, Martins AD, Cavaco JE et al (2013) Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers 1(2):e23992. https://doi.org/10.4161/tisb.23992
Li Z, Gao L, Tang H et al (2013) Peripheral effects of nesfatin-1 on glucose homeostasis. PLoS One. https://doi.org/10.1371/journal.pone.0071513
Aziz NM, Kamel MY, Mohamed MS, Ahmed SM (2018) Antioxidant, anti-inflammatory, and anti-apoptotic effects of zinc supplementation in testes of rats with experimentally induced diabetes. Appl Physiol Nutr Metab 43(10):1010–1018. https://doi.org/10.1139/apnm-2018-0070
Vignera LS, Condorell R, Vicari E, Dagata R, Calogero EA (2012) Diabetes mellitus and sperm parameters. J Androl 33:145–153
Wiernsperger NF (2003) Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabetes Metab 29(6):579–585. https://doi.org/10.1016/S1262-3636(07)70072-1
Murphy MP (1999) Nitric oxide and cell death. Biochim Biophys Acta 1411(C):401–414. https://doi.org/10.1080/15216540152845993
Andric SA, Janjic MM, Stojkov NJ, Kostic TS (2010) Testosterone-induced modulation of nitric oxide-cgmp signaling pathway and androgenesis in the rat Leydig cells1. Biol Reprod 83(3):434–442. https://doi.org/10.1095/biolreprod.110.083626
Ergün A, Köse SK, Aydos K, Ata A, Avci A (2007) Correlation of seminal parameters with serum lipid profile and sex hormones. Syst Biol Reprod Med 53(1):21–23
Padrón RS, Más J, Zamora R, Riverol F, Licea M, Mallea L, Rodríguez J (1989) Lipids and testicular function. Int Urol Nephrol 21(5):515–519
Oliveira PF, Martins AD, Moreira AC, Cheng CY, Alves MG (2015) The warburg effect revisited-lesson from the Sertoli cell. Med Res Rev 35(1):126–151. https://doi.org/10.1002/med.21325
Acknowledgements
The authors are highly thankful to the Interdisciplinary School of Life Sciences (ISLS) BHU Varanasi for providing instrumentation facility. AR also acknowledges the Centre of Advanced Study (CAS) Zoology, Banaras Hindu University, for financial assistance in the form of fellowship. AR designed and performed the experiment. AR and AK wrote the manuscript draft. MC and TY corrected and edited the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no conflict of interest.
Ethical approval
The present study was approved by the institutional animal ethical committee of Banaras Hindu University following the relevant guidelines and regulations. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Institutional Animal Ethics Committee (F.Sc/88/IAEC/2016-17/63)). This article does not contain any studies with human participants performed by any of the authors.
Informed consent
No informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranjan, A., Choubey, M., Yada, T. et al. Nesfatin-1 ameliorates type-2 diabetes-associated reproductive dysfunction in male mice. J Endocrinol Invest 43, 515–528 (2020). https://doi.org/10.1007/s40618-019-01136-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01136-0