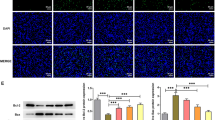
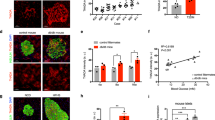
Abstract
Type 1 diabetes mellitus (T1DM) is caused by pancreatic β-cell dysfunction and apoptosis, with consequent severe insulin deficiency. Thus, β-cell protection may be a primary target in the treatment of T1DM. Evidence has demonstrated that defective mitochondrial function plays an important role in pancreatic β-cell dysfunction and apoptosis; however, the fundamental effect of mitochondrial complex I action on β-cells and T1DM remains unclear. In the current study, the pancreas protective effect of complex I inhibitor rotenone (ROT) and its potential mechanism were assessed in a streptozotocin (STZ)-induced mouse model of T1DM and in cultured mouse pancreatic β-cell line, Min6. ROT treatment exerted a hypoglycemic effect, restored the insulin level, and decreased inflammation and cell apoptosis in the pancreas. In vitro experiments also showed that ROT decreased STZ- and inflammatory cytokines-induced β-cell apoptosis. These protective effects were accompanied by attenuation of reactive oxygen species, increased mitochondrial membrane potential, and upregulation of transcriptional coactivator PPARα coactivator 1α (PGC-1α)-controlled mitochondrial biogenesis. These findings suggest that mitochondrial complex I inhibition may represent a promising strategy for β-cell protection in T1DM.







Similar content being viewed by others
References
Chiang JL, Kirkman MS, Laffel LM, Peters AL, Sourcebook A (2014) Type 1 diabetes. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 37(7):2034–2054. https://doi.org/10.2337/dc14-1140
Bluestone JA, Herold K, Eisenbarth G (2010) Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464(7293):1293–1300. https://doi.org/10.1038/nature08933
Supale S, Li N, Brun T, Maechler P (2012) Mitochondrial dysfunction in pancreatic beta cells. Trends Endocrinol Metab 23(9):477–487. https://doi.org/10.1016/j.tem.2012.06.002
Lee JW, Kim WH, Lim JH, Song EH, Song J, Choi KY, Jung MH (2009) Mitochondrial dysfunction: glucokinase downregulation lowers interaction of glucokinase with mitochondria, resulting in apoptosis of pancreatic beta-cells. Cell Signal 21(1):69–78. https://doi.org/10.1016/j.cellsig.2008.09.015
Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS (2008) Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab 8(4):318–324. https://doi.org/10.1016/j.cmet.2008.08.013
Nishikawa T, Araki E (2007) Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 9(3):343–353. https://doi.org/10.1089/ars.2007.9.ft-19
Gurzov EN, Eizirik DL (2011) Bcl-2 proteins in diabetes: mitochondrial pathways of beta-cell death and dysfunction. Trends Cell Biol 21(7):424–431. https://doi.org/10.1016/j.tcb.2011.03.001
Raza H, Prabu SK, John A, Avadhani NG (2011) Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. Int J Mol Sci 12(5):3133–3147. https://doi.org/10.3390/ijms12053133
Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N (2004) Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 37(6):755–767. https://doi.org/10.1016/j.freeradbiomed.2004.05.034
Gerber PA, Rutter GA (2017) The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal 26(10):501–518. https://doi.org/10.1089/ars.2016.6755
St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277(47):44784–44790. https://doi.org/10.1074/jbc.M207217200
Xu X, Arriaga EA (2009) Qualitative determination of superoxide release at both sides of the mitochondrial inner membrane by capillary electrophoretic analysis of the oxidation products of triphenylphosphonium hydroethidine. Free Radic Biol Med 46(7):905–913. https://doi.org/10.1016/j.freeradbiomed.2008.12.019
Bleier L, Wittig I, Heide H, Steger M, Brandt U, Drose S (2015) Generator-specific targets of mitochondrial reactive oxygen species. Free Radic Biol Med 78:1–10. https://doi.org/10.1016/j.freeradbiomed.2014.10.511
Xiong N, Long X, Xiong J, Jia M, Chen C, Huang J, Ghoorah D, Kong X, Lin Z, Wang T (2012) Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson's disease models. Crit Rev Toxicol 42(7):613–632. https://doi.org/10.3109/10408444.2012.680431
Hou WL, Yin J, Alimujiang M, Yu XY, Ai LG, Bao YQ, Liu F, Jia WP (2018) Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. J Cell Mol Med 22(2):1316–1328. https://doi.org/10.1111/jcmm.13432
Ai Q, Jing Y, Jiang R, Lin L, Dai J, Che Q, Zhou D, Jia M, Wan J, Zhang L (2014) Rotenone, a mitochondrial respiratory complex I inhibitor, ameliorates lipopolysaccharide/D-galactosamine-induced fulminant hepatitis in mice. Int Immunopharmacol 21(1):200–207. https://doi.org/10.1016/j.intimp.2014.04.028
Yin J, Xia W, Wu M, Zhang Y, Huang S, Zhang A, Jia Z (2019) Inhibition of mitochondrial complex I activity attenuates neointimal hyperplasia by inhibiting smooth muscle cell proliferation and migration. Chem Biol Interact 304:73–82. https://doi.org/10.1016/j.cbi.2019.03.002
Hua H, Ge X, Wu M, Zhu C, Chen L, Yang G, Zhang Y, Huang S, Zhang A, Jia Z (2018) Rotenone protects against acetaminophen-induced kidney injury by attenuating oxidative stress and inflammation. Kidney Blood Press Res 43(4):1297–1309. https://doi.org/10.1159/000492589
Ding W, Xu C, Wang B, Zhang M (2015) Rotenone attenuates renal injury in aldosterone-infused rats by inhibiting oxidative stress, mitochondrial dysfunction, and inflammasome activation. Med Sci Monit 21:3136–3143. https://doi.org/10.1038/nature08933
Sun Y, Zhang Y, Zhao D, Ding G, Huang S, Zhang A, Jia Z (2014) Rotenone remarkably attenuates oxidative stress, inflammation, and fibrosis in chronic obstructive uropathy. Mediators Inflamm 2014:670106. https://doi.org/10.1155/2014/670106
Zhang W, Sha Y, Wei K, Wu C, Ding D, Yang Y, Zhu C, Zhang Y, Ding G, Zhang A, Jia Z, Huang S (2018) Rotenone ameliorates chronic renal injury caused by acute ischemia/reperfusion. Oncotarget 9(36):24199–24208. https://doi.org/10.18632/oncotarget.24733
Zhang A, Jia Z, Wang N, Tidwell TJ, Yang T (2011) Relative contributions of mitochondria and NADPH oxidase to deoxycorticosterone acetate-salt hypertension in mice. Kidney Int 80(1):51–60. https://doi.org/10.1038/ki.2011.29
Ozay EI, Sherman HL, Mello V, Trombley G, Lerman A, Tew GN, Yadava N, Minter LM (2018) Rotenone treatment reveals a role for electron transport complex I in the subcellular localization of key transcriptional regulators during T helper cell differentiation. Front Immunol 9:1284. https://doi.org/10.3389/fimmu.2018.01284
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Johnson ME, Bobrovskaya L (2015) An update on the rotenone models of Parkinson's disease: their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology 46:101–116. https://doi.org/10.1016/j.neuro.2014.12.002
Heinz S, Freyberger A, Lawrenz B, Schladt L, Schmuck G, Ellinger-Ziegelbauer H (2017) Mechanistic investigations of the mitochondrial complex I inhibitor rotenone in the context of pharmacological and safety evaluation. Sci Rep 7:45465. https://doi.org/10.1038/srep45465
Bove J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson's disease. NeuroRx 2(3):484–494. https://doi.org/10.1602/neurorx.2.3.484
Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT (2003) Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci 23(34):10756–10764. https://doi.org/10.1523/JNEUROSCI.23-34-10756.2003
Greenamyre JT, Betarbet R, Sherer TB (2003) The rotenone model of Parkinson's disease: genes, environment and mitochondria. Parkinsonism Relat Disord 9(Suppl 2):S59–S64. https://doi.org/10.1016/S1353-8020(03)00023-3
Furman BL (2015) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 70:5 47 41 – 20. https://doi.org/10.1002/0471141755.ph0547s70
Zhang H, Gong G, Wang P, Zhang Z, Kolwicz SC, Rabinovitch PS, Tian R, Wang W (2018) Heart specific knockout of Ndufs4 ameliorates ischemia reperfusion injury. J Mol Cell Cardiol 123:38–45. https://doi.org/10.1016/j.yjmcc.2018.08.022
Ling S, Shan Q, Liu P, Feng T, Zhang X, Xiang P, Chen K, Xie H, Song P, Zhou L, Liu J, Zheng S, Xu X (2017) Metformin ameliorates arsenic trioxide hepatotoxicity via inhibiting mitochondrial complex I. Cell Death Dis 8(11):e3159. https://doi.org/10.1038/cddis.2017.482
Mulder H (2017) Transcribing beta-cell mitochondria in health and disease. Mol Metab 6(9):1040–1051. https://doi.org/10.1016/j.molmet.2017.05.014
Li PA, Hou X, Hao S (2017) Mitochondrial biogenesis in neurodegeneration. J Neurosci Res 95(10):2025–2029. https://doi.org/10.1002/jnr.24042
Scarpulla RC (2002) Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta 1576(1–2):1–14. https://doi.org/10.1016/s0167-4781(02)00343-3
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34(3):267–273. https://doi.org/10.1038/ng1180
Leonard S, Tobin LM, Findlay JB (2017) The signalling mechanisms of a novel mitochondrial complex I inhibitor prevent lipid accumulation and attenuate TNF-alpha-induced insulin resistance in vitro. Eur J Pharmacol 800:1–8. https://doi.org/10.1016/j.ejphar.2017.01.007
Wu M, Li S, Yu X, Chen W, Ma H, Shao C, Zhang Y, Zhang A, Huang S, Jia Z (2019) Mitochondrial activity contributes to impaired renal metabolic homeostasis and renal pathology in STZ-induced diabetic mice. Am J Physiol Renal Physiol. https://doi.org/10.1152/ajprenal.00076.2019
Yuan S, Liu X, Zhu X, Qu Z, Gong Z, Li J, Xiao L, Yang Y, Liu H, Sun L, Liu F (2018) The role of TLR4 on PGC-1alpha-mediated oxidative stress in tubular cell in diabetic kidney disease. Oxid Med Cell Longev 2018:6296802. https://doi.org/10.1155/2018/6296802
Ma S, Feng J, Zhang R, Chen J, Han D, Li X, Yang B, Li X, Fan M, Li C, Tian Z, Wang Y, Cao F (2017) SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid Med Cell Longev 2017:4602715. https://doi.org/10.1155/2017/4602715
Rowe GC, Raghuram S, Jang C, Nagy JA, Patten IS, Goyal A, Chan MC, Liu LX, Jiang A, Spokes KC, Beeler D, Dvorak H, Aird WC, Arany Z (2014) PGC-1alpha induces SPP1 to activate macrophages and orchestrate functional angiogenesis in skeletal muscle. Circ Res 115(5):504–517. https://doi.org/10.1161/CIRCRESAHA.115.303829
Acknowledgements
This work was supported by grants from the National Natural Science Foundation (Nos. 81800599, 81830020, 81873599, 81600352, 81670678, 81530023), the Natural Science Foundation of Jiangsu Province (No. BK20180145), the China Postdoctoral Science Foundation (No. 2018M632342) and Nanjing City Key Medical Research Project (No. ZKX18039).
Author information
Authors and Affiliations
Contributions
Z.J., Y. Z., S.H., and A.Z coordinated and oversaw the study. M.W., W.C., and S.Z. performed the experiments, collected the samples, and analyzed the data. M.W., Y. Z., and Z.J. wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethical approval
All animal experiments are in accordance with International Guidelines and Protocols and approved by the Nanjing Medical University Institutional Animal Care and Use Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, M., Chen, W., Zhang, S. et al. Rotenone protects against β-cell apoptosis and attenuates type 1 diabetes mellitus. Apoptosis 24, 879–891 (2019). https://doi.org/10.1007/s10495-019-01566-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-019-01566-4