Abstract
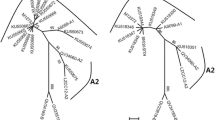
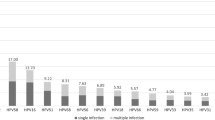
The high-risk human papillomaviruses (HR-HPVs) are involved in the development of cervical cancer. Nevertheless, there are differences in the oncogenic potential among them. HPV-16 and HPV-18 are associated with approximately 70% of cancer worldwide, and both types are the most extensively studied HR-HPV. Great variations in the prevalence of HR-HPV have been described in different countries. The impact of these variations on the epidemiology of lesions and cervical cancer is currently unknown. A high prevalence of HPV-66 has been detected in Chile. Here, we have analyzed the genetic variability of the L1 gene from HPV-66-infected Chilean women. Higher order interactions between identified mutations were analyzed by co-variation and cluster analyses. Antigenic-index alterations following L1 mutations and B-cell epitopes were predicted by BcePred algorithm. HPV-66 L1 sequences clustered phylogenetically into two main clades. The genetic variability in the HPV-66 L1 gene involved thirty nucleotide changes. Four of these were for the first time identified in this study. Some of these variants are embedded in the B-cell epitope regions. Amino acid homology in the immunodominant epitopes of HPV-66 L1 protein (DE, FG and H1 loops) was 42.9–59.1% and 28.6–68.9% compared with HPV-16 and HPV-18, respectively. The results of this research suggest that the neutralizing epitopes of HPV-66 are antigenically different compared to HPV-16 and HPV-18. Our findings show the need to perform new structural and immunological studies on HPV-66 L1 protein to evaluate the cross-protection conferred by current HPV vaccines.




Similar content being viewed by others
References
de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H (2004) Classification of papillomaviruses. Virology 324(1):17–27. https://doi.org/10.1016/j.virol.2004.03.033
Bernard HU, Burk RD, Chen Z, van Doorslaer K, zur Hausen H, de Villiers EM (2010) Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401(1):70–79. https://doi.org/10.1016/j.virol.2010.02.002
International Human Papillomavirus (HPV) Reference Center (2019) http://www.hpvcenter.se/html/refclones.html. Accessed 01 March 2019
zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2(5):342–350. https://doi.org/10.1038/nrc798
Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348(6):518–527. https://doi.org/10.1056/NEJMoa021641
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, WHO-IARC Monograph Working Group (2009) A review of human carcinogens-part B: biological agents. Lancet Oncol 10(4):321–322
Cerqueira DM, Camara GN, da Cruz MR, Silva EO, de Brigido MM, Carvalho LG, Martins CR (2003) Variants of human papillomavirus types 53, 58 and 66 identified in Central Brazil. Virus Genes 26(1):83–87
Prado JC, Calleja-Macias IE, Bernard HU, Kalantari M, Macay SA, Allan B, Williamson AL, Chung LP, Collins RJ, Zuna RE, Dunn ST, Ortiz-Lopez R, Barrera-Saldana HA, Cubie HA, Cuschieri K, von Knebel-Doeberitz M, Sanchez GI, Bosch FX, Villa LL (2005) Worldwide genomic diversity of the human papillomaviruses-53, 56, and 66, a group of high-risk HPVs unrelated to HPV-16 and HPV-18. Virology 340(1):95–104. https://doi.org/10.1016/j.virol.2005.06.024
Castro MM, Farias IP, Borborema-Santos CM, Correia G, Astolfi-Filho S (2011) Prevalence of human papillomavirus (HPV) type 16 variants and rare HPV types in the central Amazon region. Genet Mol Res 10(1):186–196. https://doi.org/10.4238/vol10-1gmr992
Wyant PS, Cerqueira DM, Moraes DS, Leite JP, Martins CR, de Macedo Brigido M, Raiol T (2011) Phylogeny and polymorphism in the long control region, E6, and L1 of human papillomavirus types 53, 56, and 66 in central Brazil. Int J Gynecol Cancer 21(2):222–229. https://doi.org/10.1097/IGC.0b013e318208c73d
Cento V, Rahmatalla N, Ciccozzi M, Lo Presti A, Perno CF, Ciotti M (2012) Human papillomaviruses 53 and 66: clinical aspects and genetic analysis. Virus Res 163(1):212–222. https://doi.org/10.1016/j.virusres.2011.09.032
Chouhy D, Mamprín D’Andrea R, Iglesias M, Messina A, Ivancovich J, Cerda B, Galimberti D, Bottai H, Giri A (2013) Prevalence of human papillomavirus infection in Argentinean women attending two different hospitals prior to the implementation of the National vaccination program. J Med Virol 85(4):655–666. https://doi.org/10.1002/jmv.23509
Yamada T, Manos MM, Peto J, Greer CE, Munoz N, Bosch FX, Wheeler CM (1997) Human papillomavirus type 16 sequence variation in cervical cancers: a worldwide perspective. J Virol 71(3):2463–2472
Cento V, Ciccozzi M, Ronga L, Perno CF, Ciotti M (2009) Genetic diversity of human papillomavirus type 16 E6, E7, and L1 genes in Italian women with different grades of cervical lesions. J Med Virol 81(9):1627–1634. https://doi.org/10.1002/jmv.21552
Calleja-Macias IE, Kalantari M, Allan B, Williamson AL, Chung LP, Collins RJ, Zuna RE, Dunn ST, Ortiz-Lopez R, Barrera-Saldaña HA, Cubie HA, Cuschieri K, Villa LL, Bernard HU (2005) Papillomavirus subtypes are natural and old taxa: phylogeny of the human papillomavirus (HPV) types 44/55 and 68a/b. J Virol 79(10):6565–6569. https://doi.org/10.1128/JVI.79.10.6565-6569.2005
Calleja-Macias IE, Villa LL, Prado JC, Kalantari M, Allan B, Williamson AL, Chung LP, Collins RJ, Zuna RE, Dunn ST, Chu TY, Cubie HA, Cuschieri K, von Knebel-Doeberitz M, Martins CR, Sanchez GI, Bosch FX, Munoz N, Bernard HU (2005) Worldwide genomic diversity of the high-risk human papillomavirus types 31, 35, 52, and 58, four close relatives of human papillomavirus type 16. J Virol 79(21):13630–13640. https://doi.org/10.1128/JVI.79.21.13630-13640.2005
Hibbitts S, Rieck GC, Hart K, Powell NG, Beukenholdt R, Dallimore N, McRea J, Hauke A, Tristram A, Fiander AN (2006) Human papillomavirus infection: an Anonymous Prevalence Study in South Wales, UK. Br J Cancer 95(2):226–232. https://doi.org/10.1038/sj.bjc.6603245
Giambi C, Donati S, Carozzi F, Salmaso S, Declich S, Ciofi Degli Atti M, Ronco G, Alibrandi M, Brezzi S, Collina N, Franchi D, Lattanzi A, Minna M, Nannini R, Barretta E, Burroni E, Gillio-Tos A, Macallini V, Pierotti P, Bella A (2013) A cross-sectional study to estimate high-risk human papillomavirus prevalence and type distribution in Italian women aged 18–26 years. BMC Infect Dis 13:74. https://doi.org/10.1186/1471-2334-13-74
Menegazzi P, Barzon L, Palù G, Reho E, Tagliaferro L (2009) Human Papillomavirus Type Distribution and Correlation with Cyto-Histological Patterns in Women from the South of Italy. Infect Dis Obstet Gynecol 2009:198425. https://doi.org/10.1155/2009/198425
Jiang Y, Brassard P, Severini A, Mao Y, Li A, Laroche J, Chatwood S, Corriveau A, Kandola K, Hanley B, Sobol I, Ar-Rushdi M, Johnson G, Lo J, Ratnam S, Wong T, Demers A, Jayaraman G, Totten S, Morrison H (2013) The prevalence of human papillomavirus and its impact on cervical dysplasia in Northern Canada. Infect Agent Cancer 8:25. https://doi.org/10.1186/1750-9378-8-25
Mbulawa ZZA, van Schalkwyk C, Hu NC, Meiring TL, Barnabas S, Dabee S, Jaspan H, Kriek JM, Jaumdally SZ, Muller E, Bekker LG, Lewis DA, Dietrich J, Gray G, Passmore JS, Williamson AL (2018) High human papillomavirus (HPV) prevalence in South African adolescents and young women encourages expanded HPV vaccination campaigns. PLoS ONE 13(1):e0190166. https://doi.org/10.1371/journal.pone.0190166
Zhao FH, Zhu FC, Chen W, Li J, Hu YM, Hong Y, Zhang YJ, Pan QJ, Zhu JH, Zhang X, Chen Y, Tang H, Zhang H, Durand C, Datta SK, Struyf F, Bi D (2014) Baseline prevalence and type distribution of human papillomavirus in healthy Chinese women aged 18-25 years enrolled in a clinical trial. Int J Cancer 135(11):2604–2611. https://doi.org/10.1002/ijc.28896
Bonde J, Rebolj M, Ejegod D, Preisler S, Lynge E, Rygaard C (2014) HPV prevalence and genotype distribution in a population-based split-sample study of well-screened women using CLART HPV2 Human Papillomavirus genotype microarray system. BMC Infect Dis 14:413. https://doi.org/10.1186/1471-2334-14-413
Roksandić-Krizan I, Bosnjak Z, Perić M, Durkin I, Atalić VZ, Vuković D (2013) Distribution of genital human papillomavirus (HPV) genotypes in Croatian women with cervical intraepithelial neoplasia (CIN)—a pilot study. Coll Antropol 37(4):1179–1183
Malagón T, Drolet M, Boily M-C, Franco EL, Jit M, Brisson J, Brisson M (2012) Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12(10):781–789. https://doi.org/10.1016/S1473-3099(12)70187-1
Balanda M, Quiero A, Vergara N, Espinoza G, Martín HS, Rojas G, Ramírez E (2016) Prevalence of human papillomavirus infection among women presenting for cervical cancer screening in Chile, 2014–2015. Med Microbiol Immunol 205(6):585–594. https://doi.org/10.1007/s00430-016-0473-y
Vergara N, Espinoza G, Balanda M, Quiero A, Hidalgo W, San Martín H, Ramírez A, Ramírez E (2017) Prevalence of Human Papillomavirus infection among Chilean women from 2012 to 2016. J Med Virol 89(9):1646–1653. https://doi.org/10.1002/jmv.24805
Coutlée F, Gravitt P, Kornegay J, Hankins C, Richardson H, Lapointe N, Voyer H, Franco E (2002) Use of PGMY primers in L1 consensus PCR improves detection of human papillomavirus DNA in genital samples. J Clin Microbiol 40(3):902–907. https://doi.org/10.1128/JCM.40.3.902-907.2002
Montanheiro PA, Penalva de Oliveira AC, Posada-Vergara MP, Milagres AC, Tauil C, Marchiori PE, Duarte AJ, Casseb J (2005) Human T-cell lymphotropic virus type I (HTLV-I) proviral DNA viral load among asymptomatic patients and patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. Braz J Med Biol Res 38(11):1643–1647. https://doi.org/10.1590/S0100-879X2005001100011
World Health Organization (2010) Human papillomavirus laboratory manual, First edition. WHO/IVB/10.12. http://whqlibdoc.who.int/hq/2010/WHO_IVB_10.12_eng.pdf. Accessed 16 August 2017
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Saha S, Raghava GPS (2004) BcePred: prediction of continuous B-Cell epitopes in antigenic sequences using physico-chemical properties. In: Nicosia G, Cutello V, Bentley PJ, Timis J (eds) ICARIS, LNCS 3239. Springer, pp 197–204. http://crdd.osdd.net/raghava/bcepred/. Accessed 12 November 2018
Carter JJ, Wipf GC, Madeleine MM, Schwartz SM, Koutsky LA, Galloway DA (2006) Identification of human papillomavirus type 16 L1 surface loops required for neutralization by human sera. J Virol 80(10):4664–4672. https://doi.org/10.1128/JVI.80.10.4664-4672.2006
Xia L, Xian Y, Wang D, Chen Y, Huang X, Bi X, Yu H, Fu Z, Liu X, Li S, An Z, Luo W, Zhao Q, Xia N (2016) A human monoclonal antibody against HPV16 recognizes an immunodominant and neutralizing epitope partially overlapping with that of H16.V5. Sci Rep 6:19042. https://doi.org/10.1038/srep19042
Bishop B, Dasgupta J, Klein M, Garcea RL, Christensen ND, Zhao R, Chen XS (2007) Crystal structures of four types of human papillomavirus L1 capsid proteins: understanding the specificity of neutralizing monoclonal antibodies. J Biol Chem 282(43):31803–31811. https://doi.org/10.1074/jbc.M706380200
Bi Q, Zhang L, Zhao Z, Mu X, Zhang M, Wang P (2015) Human papillomavirus prevalence and genotypes distribution among female outpatients in Qingdao, East China. J Med Virol 87(12):2114–2121. https://doi.org/10.1002/jmv.24281
De Vuyst H, Steyaert S, Van Renterghem L, Claeys P, Muchiri L, Sitati S, Vansteelandt S, Quint W, Kleter B, Van Marck E, Temmerman M (2003) Distribution of human papillomavirus in a family planning population in Nairobi, Kenya. Sex Transm Dis 30(2):137–142
Meyer T, Arndt R, Beckmann ER, Padberg B, Christophers E, Stockfleth E (2001) Distribution of HPV 53, HPV 73 and CP8304 in genital epithelial lesions with different grades of dysplasia. Int J Gynecol Cancer 11(3):198–204. https://doi.org/10.1046/j.1525-1438.2001.01009.x
Pastrana DV, Vass WC, Lowy DR, Schiller JT (2001) NHPV16VLP vaccine induces human antibodies that neutralize divergent variants of HPV16. Virology 279(1):361–369. https://doi.org/10.1006/viro.2000.0702
Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC (2000) Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell 5(3):557–567. https://doi.org/10.1016/S1097-2765(00)80449-9
Christensen N, Dillner J, Eklund C, Carter J, Wipf G, Reed C, Cladel N, Galloway D (1996) Surface Conformational and Linear Epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223(1):174–184. https://doi.org/10.1006/viro.1996.0466
Christensen N, Cladel N, Reed C, Budgeon L, Embers M, Skulsky D, McClements W, Ludmerer S, Jansen K (2001) Hybrid papillomavirus L1 molecules assemble into virus-like particles that reconstitute conformational epitopes and induce neutralizing antibodies to distinct HPV types. Virology 291(2):324–334. https://doi.org/10.1006/viro.2001.1220
Carter JJ, Wipf GC, Benki SF, Christensen ND, Galloway DA (2003) Identification of a human papillomavirus type 16-specific epitope on the C-terminal arm of the major capsid protein L1. J Virol 77(21):11625–11632. https://doi.org/10.1128/JVI.77.21.11625-11632.2003
Wang Z, Christensen N, Schiller JT, Dillner J (1997) A monoclonal antibody against intact human papillomavirus type 16 capsids blocks the serological reactivity of most human sera. J Gen Virol 78(9):2209–2215. https://doi.org/10.1099/0022-1317-78-9-2209
Wang X, Wang Z, Christensen ND, Dillner J (2003) Mapping of human serum-reactive epitopes in virus-like particles of human papillomavirus types 16 and 11. Virology 311(1):213–221. https://doi.org/10.1016/S0042-6822(03)00179-X
Arbyn M, Tommasino M, Depuydt Ch, Dillner J (2014) Are 20 human papillomavirus types causing cervical cancer? J Pathol 234(4):431–435. https://doi.org/10.1002/path.4424
de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menéndez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andújar M, Castellsagué X, Sánchez GI, Nowakowski AM, Bornstein J, Muñoz N, Bosch FX (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11(11):1048–1056. https://doi.org/10.1016/S1470-2045(10)70230-8
Geraets D, Alemany L, Guimera N, de Sanjose S, de Koning M, Molijn A, Jenkins D, Bosch X, Quint W (2012) Detection of rare and possibly carcinogenic human papillomavirus genotypes as single infections in invasive cervical cancer. J Pathol 228(4):534–543. https://doi.org/10.1002/path.4065
Serra IGSS, Araujo ED, Barros GS, Santos FLSG, Gurgel RQ, Batista MVA (2018) Prevalence of human papillomavirus types associated with cervical lesions in Sergipe state, Northeastern Brazil: high frequency of a possibly carcinogenic type. Epidemiol Infect 146(9):1184–1193. https://doi.org/10.1017/S095026881800105X
Halec G, Alemany L, Lloveras B, Schmitt M, Alejo M, Bosch FX, Tous S, Klaustermeier JE, Guimerà N, Grabe N, Lahrmann B, Gissmann L, Quint W, Bosch FX, de Sanjose S, Pawlita M (2014) Pathogenic role of the eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J Pathol 234(4):441–451. https://doi.org/10.1002/path.4405
Acknowledgements
We thank Deyanira Vidal and Francisco Roldán for supporting the DNA purification and HPV typing assays; and Vivian Gómez and María Ibáñez for technical help with the sequencing of L1 gene.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
Six public health care centers and their referral hospital participated in this study using the infrastructure, personnel and protocols already in place under the national cervical cancer prevention program. The study protocol was approved by the Ethics Committee of the Servicio de Salud Metropolitano Central.
Informed consent
Women were invited to participate through an outreach campaign in the catchment area of each health center and, if interested, received an appointment with the health center midwife. Eligible women who agreed to participate signed an informed consent form and entered the study.
Additional information
Edited by: Matthias J. Reddehase.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balanda, M., Fernández, J., Vergara, N. et al. Genetic variability of human papillomavirus type 66 L1 gene among women presenting for cervical cancer screening in Chile. Med Microbiol Immunol 208, 757–771 (2019). https://doi.org/10.1007/s00430-019-00621-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-019-00621-w