Abstract
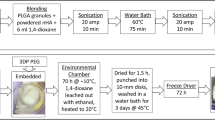
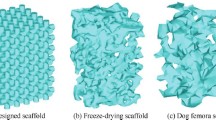
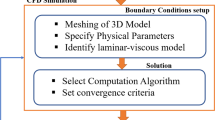
Freeze-casting is a popular method to produce biomaterial scaffolds with highly porous structures. The pore structure of freeze-cast biomaterial scaffolds is influenced by processing parameters but has mostly been controlled experimentally. A mathematical model integrating Computational Fluid Dynamics with Population Balance Model was developed to predict average pore size (APS) of 3D porous chitosan-alginate scaffolds and to assess the influence of the geometrical parameters of mold on scaffold pore structure. The model predicted the crystallization pattern and APS for scaffolds cast in different diameter molds and filled to different heights. The predictions demonstrated that the temperature gradient and solidification pattern affect ice crystal nucleation and growth, subsequently influencing APS homogeneity. The predicted APS compared favorably with APS measurements from a corresponding experimental dataset, validating the model. Sensitivity analysis was performed to assess the response of the APS to the three geometrical parameters of the mold: well radius; solution fill height; and spacing between wells. The pore size was most sensitive to the distance between the wells and least sensitive to solution height. This validated model demonstrates a method for optimizing the APS of freeze-cast biomaterial scaffolds that could be applied to other compositions or applications.









Similar content being viewed by others
References
Bose, S., M. Roy, and A. Bandyopadhyay. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 30:546–554, 2012.
Canonsburg, T. D. Population Balance Model Theory. ANSYS Fluent User Manual. Canonsburg: ANSYS, 2009.
Costa, C. B. B., M. R. W. Maciel, and R. M. Filho. Considerations on the crystallization modeling: population balance solution. Comput. Chem. Eng. 31:206–218, 2007.
Deville, S., E. Maire, A. Lasalle, A. Bogner, C. Gauthier, J. Leloup, and C. Guizard. In situ X-ray radiography and tomography observations of the solidification of aqueous alumina particle suspensions-part I: initial instants. J. Am. Ceram. Soc. 92:2489–2496, 2009.
Enrique, D., P. Jos, A. Sobral, and C. Norte. The effect of processing parameters and solid concentration on the microstructure and pore architecture of gelatin-chitosan scaffolds produced by freeze-drying. Mater. Res. 19:839–845, 2016.
Florczyk, S. J., F. M. Kievit, K. Wang, A. E. Erickson, R. G. Ellenbogen, and M. Zhang. 3D porous chitosan–alginate scaffolds promote proliferation and enrichment of cancer stem-like cells. J. Mater. Chem. B 4:6326–6334, 2016.
Florczyk, S. J., D.-J. J. Kim, D. L. Wood, and M. Zhang. Influence of processing parameters on pore structure of 3D porous chitosan-alginate polyelectrolyte complex scaffolds. J. Biomed. Mater. Res. Part A 98A:614–620, 2011.
Florczyk, S. J., M. Leung, Z. Li, J. I. Huang, R. A. Hopper, and M. Zhang. Evaluation of three-dimensional porous chitosan-alginate scaffolds in rat calvarial defects for bone regeneration applications. J. Biomed. Mater. Res. Part A 101:2974–2983, 2013.
Florczyk, S. J., G. Liu, F. M. Kievit, A. M. Lewis, J. D. Wu, and M. Zhang. 3D porous chitosan-alginate scaffolds: a new matrix for studying prostate cancer cell-lymphocyte interactions in vitro. Adv. Healthc. Mater. 1:590–599, 2012.
Hottot, A., S. Vessot, and J. Andrieu. Freeze drying of pharmaceuticals in vials: influence of freezing protocol and sample configuration on ice morphology and freeze-dried cake texture. Chem. Eng. Process. Process Intensif. 46:666–674, 2007.
Hsu, S., S. W. Whu, S.-C. Hsieh, C.-L. Tsai, D. C. Chen, and T.-S. Tan. Evaluation of chitosan-alginate-hyaluronate complexes modified by an RGD-containing protein as tissue-engineering scaffolds for cartilage regeneration. Artif. Organs 28:693–703, 2004.
Husmann, A., K. Pawelec, C. Burdett, S. Best, and R. Cameron. Numerical simulations to determine the influence of mould design on ice-templated scaffold structures. J. Biomed. Eng. Inform. 1:47, 2015.
Ikada, Y. Tissue Engineering : Fundamentals and Applications. Cambridge/Amsterdam: Academic Press/Elsevier, p. 469, 2006.
Khalifa, A. Natural convective heat transfer coefficient ? A review I. Isolated vertical and horizontal surfaces. Energy Convers. Manag. 42:491–504, 2001.
Kievit, F. M., S. J. Florczyk, M. C. Leung, K. Wang, J. D. Wu, J. R. Silber, R. G. Ellenbogen, J. S. H. Lee, and M. Zhang. Proliferation and enrichment of CD133 + glioblastoma cancer stem cells on 3D chitosan-alginate scaffolds. Biomaterials 35:9137–9143, 2014.
Langford, A., B. Bhatnagar, R. Walters, S. Tchessalov, and S. Ohtake. Drying technologies for biopharmaceutical applications: recent developments and future direction. Dry. Technol. 36:677–684, 2018.
Li, Z., M. Leung, R. Hopper, R. Ellenbogen, and M. Zhang. Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials 31:404–412, 2010.
Lian, G., S. Moore, and L. Heeney. Population balance and computational fluid dynamics modelling of ice crystallisation in a scraped surface freezer. Chem. Eng. Sci. 61:7819–7826, 2006.
Lucke, M., I. Koudous, M. Sixt, M. J. Huter, and J. Strube. Integrating crystallization with experimental model parameter determination and modeling into conceptual process design for the purification of complex feed mixtures. Chem. Eng. Res. Des. 133:264–280, 2018.
Mitchell, G. R., and A. Tojeira. Role of anisotropy in tissue engineering. Procedia Eng. 59:117–125, 2013.
Morris, G., G. Power, S. Ferguson, M. Barrett, G. Hou, and B. Glennon. Estimation of nucleation and growth kinetics of benzoic acid by population balance modeling of a continuous cooling mixed suspension, mixed product removal crystallizer. Org. Process Res. Dev. 19:1891–1902, 2015.
Mueller, S. M., S. Shortkroff, T. O. Schneider, H. A. Breinan, V. Yannas, and M. Spector. Meniscus cells seeded in type I and type II collagen-GAG matrices in vitro. Biomaterials 20:701–709, 1999.
Muzzarelli, R., M. Mehtedi, M. Mattioli-Belmonte, R. A. A. Muzzarelli, M. El Mehtedi, and M. Mattioli-Belmonte. Emerging biomedical applications of nano-chitins and nano-chitosans obtained via advanced eco-friendly technologies from marine resources. Mar. Drugs 12:5468–5502, 2014.
Muzzio, C. R., and N. G. Dini. Simulation of freezing step in vial lyophilization using finite element method. Comput. Chem. Eng. 35:2274–2283, 2011.
Parniakov, O., O. Bals, N. Lebovka, and E. Vorobiev. Pulsed electric field assisted vacuum freeze-drying of apple tissue. Innov. Food Sci. Emerg. Technol. 35:52–57, 2016.
Pawelec, K. M., A. Husmann, S. M. Best, and R. E. Cameron. Ice-templated structures for biomedical tissue repair: from physics to final scaffolds. Appl. Phys. Rev. 1:021301, 2014.
Porter, M. M., J. Mckittrick, and M. A. Meyers. Biomimetic materials by freeze casting. JOM 65:720–727, 2013.
Ramšak, M., J. Ravnik, M. Zadravec, M. Hriberšek, and J. Iljaž. Freeze-drying modeling of vial using BEM. Eng. Anal. Bound. Elem. 77:145–156, 2017.
Sheehan, P., and A. I. Liapis. Modeling of the primary and secondary drying stages of the freeze drying of pharmaceutical products in vials: numerical results obtained from the solution of a dynamic and spatially multi-dimensional lyophilization model for different operational policies. Biotechnol. Bioeng. 60:712–728, 1998.
Spillar, V., D. Dolejs, V. Clav, and S. Pillar. Calculation of time-dependent nucleation and growth rates from quantitative textural data: inversion of crystal size distribution. J. Petrol. 54:913–931, 2013.
Szilágyi, B., and Z. K. Nagy. Graphical processing unit (GPU) acceleration for numerical solution of population balance models using high resolution finite volume algorithm. Comput. Chem. Eng. 91:167–181, 2016.
Tang (Charlie), X., and M. J. Pikal. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm. Res. 21:191–200, 2004.
Venkatesan, J., I. Bhatnagar, and S.-K. Kim. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 12:300–316, 2014.
Venkatesan, J., I. Bhatnagar, P. Manivasagan, K.-H. Kang, and S.-K. Kim. Alginate composites for bone tissue engineering: a review. Int. J. Biol. Macromol. 72:269–281, 2015.
Xia, Z., X. Yu, X. Jiang, H. D. Brody, D. W. Rowe, and M. Wei. Fabrication and characterization of biomimetic collagen–apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 9:7308–7319, 2013.
Xu, K., K. Ganapathy, T. Andl, Z. Wang, J. A. Copland, R. Chakrabarti, and S. J. Florczyk. 3D porous chitosan-alginate scaffold stiffness promotes differential responses in prostate cancer cell lines. Biomaterials 217:119311, 2019.
Yamamoto, T. Computer modeling of polymer crystallization—toward computer-assisted materials’ design. Polymer (Guildf). 50:1975–1985, 2009.
Yin, K., P. Divakar, J. Hong, K. L. Moodie, J. M. Rosen, C. A. Sundback, M. K. Matthew, and U. G. K. Wegst. Freeze-cast porous chitosan conduit for peripheral nerve repair. MRS Adv. 3:1677–1683, 2018.
Zeltinger, J., J. K. Sherwood, D. A. Graham, R. Müeller, and L. G. Griffith. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 7:557–572, 2001.
Acknowledgments
The computation was performed with courtesy license from ANSYS. The production of the experimental dataset was supported by UCF start-up funding (SJF). The authors thank Dana Rowley and Kathryn Ellett for assistance with scaffold preparation and pore size measurements. The authors would like to acknowledge the use of JEOL JSM-6480 SEM at the Materials Characterization Facility (MCF), administered by Advanced Materials Processing and Analysis Center (AMPAC) of University of Central Florida.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Emmanuel Opara oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rouhollahi, A., Ilegbusi, O., Florczyk, S. et al. Effect of Mold Geometry on Pore Size in Freeze-Cast Chitosan-Alginate Scaffolds for Tissue Engineering. Ann Biomed Eng 48, 1090–1102 (2020). https://doi.org/10.1007/s10439-019-02381-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02381-3