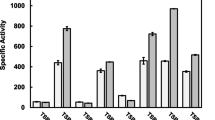
Abstract
Intracellular β-galactosidase (E.C 3.2.1.23) produced by the thermoacidophilic archeon Picrophilus torridus DSM 9790 was purified to homogeneity using a combination of DEAE Sepharose, gel filtration, hydroxyapatite and chromatofocusing chromatographies. LC–MS/MS analysis was used to confirm the identity of the purified protein. The enzyme was found to be a homotrimer, with a molecular mass of 157.0 kDa and an isoelectric point of 5.7. To our knowledge, this enzyme has the lowest pH optimum of any intracellular β-galactosidase characterized to date. Maximal activity was exhibited at acidic pH values of 5.0–5.5 and at 70 °C. The enzyme retained > 95% activity after heating to 70 °C for 1 h, or after incubation at pH 5.5 for 1 h. The enzyme may be of interest for high-temperature bioprocessing, such as in the production of lactulose. This investigation suggests that the β-galactosidase activity produced by P. torridus is potentially more useful than several enzymes already characterized for such an application.






Similar content being viewed by others
Abbreviations
- ONP:
-
Ortho-nitrophenol
- ONPG:
-
Ortho-nitrophenol-β-d-galactopyranoside
- Ylactulose :
-
Yield of lactulose to initial concentration of lactose
References
Aburto C, Castillo C, Cornejo F, Arenas-Salinas M, Vásquez C, Guerrero C, Arenas F, Illanes A, Vera C (2019) β-Galactosidase from Exiguobacterium acetylicum: cloning, expression, purification and characterization. Bioresour Technol 277:211–215
Adamczak M, Charubin D, Bednarski W (2009) Influence of reaction medium composition on enzymatic synthesis of galacto-oligosaccharides and lactulose from lactose concentrates prepared from whey permeate. Chem Pap 63:111–116
Akiyama K, Takase M, Horikoshi K, Okonogi S (2001) Production of galacto-oligosaccharides from lactose using a β-glucosidase from Thermus sp. Z-1. Biosci Biotechnol Biochem 65:438–441
Albers SV, Jonuscheit M, Dinkelaker S, Urich T, Kletzin A, Tampe R, Driessen AJM, Schleper C (2006) Production of recombinant and tagged proteins in the hyperthermophilic archaeon Sulfolobus solfataricus. Appl Environ Microbiol 72:102–111
Angelov AS (2004) Genome sequence analysis and characterization of recombinant enzymes from the thermoacidophilic archaeon Picrophilus torridus. PhD thesis
Angelov A, Futterer O, Valerius O, Braus GH, Liebl W (2005) Properties of the recombinant glucose/galactose dehydrogenase from the extreme thermoacidophile, Picrophilus torridus. FEBS J 272:1054–1062
Angelov A, Putyrski M, Liebl W (2006) Molecular and biochemical characterisation of α-glucosidase and α-mannosidase and their clustered genes from the thermoacidophilic archaeon Picrophilus torridus. J Bacteriol 188:7123–7131
Bertoldo C, Dock C, Antranikian G (2004) Thermoacidophilic microorganisms and their novel biocatalysts. Eng Life Sci 4:521–532
BIO-RAD (2004) Model 111 mini IEF cell instruction manual
Buonocore V, Sgambati O, Rosa M, Esposito E, Gambacorta A (1980) A constitutive β-galactosidase from the extreme thermoacidophile archaebacterium Caldariella acidophila: properties of the enzyme in the free state and in immobilised whole cells. J Appl Biochem 2:390–397
Carninci P, Nishiyama Y, Westover A, Itoh M, Nagaoka S, Sasaki N, Okazaki Y, Muramatsu M, Hayashizaki Y (1998) Thermostabilisation and thermoactivation of thermolabile enzymes by trehalose and its application for the synthesis of full length cDNA. PNAS 95:520–524
Chakraborti S, Sani RK, Banerjee UC, Sobti RC (2000) Purification and characterisation of a novel β-galactosidase from Bacillus sp. MTCC 3088. J Ind Microbiol Biotechnol 24:58–63
Chen YS, Lee GC, Shaw JF (2006) Gene cloning, expression, and biochemical characterisation of a recombinant trehalose synthase from Picrophilus torridus in Escherichia coli. J Agric Food Chem 54:7098–7104
Fütterer O, Angelov A, Liesegang H, Gottschalk G, Schleper C, Schepers B, Dock C, Antranikian G, Liebl W (2004) Genome sequence of Picrophilus torridus and its implications for life around pH 0. PNAS 101:9091–9096
Grogan DW (1991) Evidence that β-galactosidase of Sulfolobus solfataricus is only one of several activities of a thermostable β-d-glycosidase. Appl Environ Microbiol 57:1644–1649
Guerrero C, Vera C, Plou F, Illanes A (2011) Influence of reaction conditions on the selectivity of the synthesis of lactulose with microbial β-galactosidases. J Mol Catal B Enzym 72:206–212
Guerrero C, Vera C, Araya E, Conejeros R, Illanes A (2015) Repeated-batch operation for the synthesis of lactulose with β-galactosidase immobilized by aggregation and crosslinking. Bioresour Technol 190:122–131
Hess M, Katzer M, Antranikian G (2008) Extremely thermostable esterases from the thermoacidophilic euryarchaeon Picrophilus torridus. Extremophiles 12:351–364
Holmes ML, Scopes RK, Moritz RL, Simpson RJ, Englert C, Pfeifer F, Dyall-Smith ML (1997) Purification and analysis of an extremely halophilic β-galactosidase from Haloferax alicantei. Biochim Biophys Acta 1337:276–286
Horikoshi K (2011) Extremophiles handbook. Springer, Berlin
Hoyoux A, Jennes I, Dubois P, Genicot S, Dubail F, Francois JM, Baise E, Feller G, Gerday C (2001) Cold-adapted β-galactosidase from the Antarctic psychrophile Pseudoalteromonas haloplanktis. Appl Environ Microbiol 67:1529–1535
Juajun O, Nguyen TH, Maischberger T, Iqbal S, Haltrich D, Yamabhai M (2011) Cloning, purification, and characterisation of β-galactosidase from Bacillus licheniformis DSM 13. Appl Microbiol Biotechnol 89:645–654
Karan R, Capes MD, DasSarma P, DasSarma S (2013) Cloning, overexpression, purification, and characterisation of a polyextremophilic β-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi. BMC Biotechnol 13:11
Kim CS, Ji ES, Oh DK (2003) Expression and characterisation of Kluyveromyces lactis β-galactosidase in Escherichia coli. Biotechnol Lett 25:1769–1774
Kim YS, Park CS, Oh DK (2006) Lactulose production from lactose and fructose by a thermostable β-galactosidase from Sulfolobus solfataricus. Enzyme Microb Technol 39:903–908
Kosugi A, Murashima K, Doi RH (2002) Characterisation of two noncellulosomal subunits, ArfA and BgaA, from Clostridium cellulovorans that cooperate with the cellulosome in plant cell wall degradation. J Bacteriol 184:6859–6865
Krüger A, Schäfers C, Schröder C, Antranikian G (2018) Towards a sustainable biobased industry—highlighting the impact of extremophiles. New Biotechnol 40:144–153
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee MS (2012) Mass spectrometry handbook. Pharmaceutical science and biotechnology: practices, applications and methods. Wiley
Lee YJ, Kim CS, Oh DK (2004) Lactulose production by β-galactosidase in permeabilised cells of Kluyveromyces lactis. Appl Microbiol Biotechnol 64:787–793
Lee JH, Kim YS, Yeom SJ, Oh DK (2011) Characterisation of a glycoside hydrolase family 42 β-galactosidase from Deinococcus geothermalis. Biotechnol Lett 33:577–583
Li Y, Wang H, Lu L, Li Z, Xu X, Xiao M (2009) Purification and characterisation of a novel β-galactosidase with transglycosylation activity from Bacillus megaterium 2037-4-1. Appl Biochem Biotechnol 158:192–199
Liao XY, Zheng QW, Zhou QL, Lin JF, Guo LQ, Yun F (2016) Characterization of recombinant β-galactosidase and its use in enzymatic synthesis of lactulose from lactose and fructose. J Mol Catal B Enzym 134:253–260
Linares-Pasten JA, Andersson M, Karlsson EN (2014) Thermostable glycoside hydrolases in biorefinery technologies. Curr Biotechnol 3:26–44
Mayer J, Conrad J, Klaiber I, Lutz-Wahl S, Beifuss U, Fischer L (2004) Enzymatic production and complete nuclear magnetic resonance assignment of the sugar lactulose. J Agric Food Chem 52:6983–6990
Naessens M, Vandamme EJ (2003) Multiple forms of microbial enzymes. Biotechnol Lett 25:1119–1124
Nagy Z, Kiss T, Szentirmai A, Biro S (2001) β-Galactosidase of Penicillium chrysogenum: production, purification, and characterisation of the enzyme. Protein Expr Purif 21:24–29
Nooshkam M, Babazedeh A, Jooyandeh H (2018) Lactulose: Properties, techno-functional food applications, and food grade delivery system. Trends Food Sci Technol 80:23–34
O’Connell S, Walsh G (2007) Purification and properties of a β-galactosidase with potential application as a digestive supplement. Appl Biochem Biotechnol 141:1–14
O’Connell S, Walsh G (2010) A novel acid-stable, acid-active β-galactosidase potentially suited to the alleviation of lactose intolerance. Appl Microbiol Biotechnol 86:517–524
Onishi N, Tanaka T (1995) Purification and characterisation of a novel thermostable galacto-oligosaccharide-producing β-galactosidase from Sterigmatomyces elviae CBS8119. Appl Environ Microbiol 61:4026–4030
Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551–3567
Pisani FM, Rella R, Raia CA, Rozzo C, Nucci R, Gambacorta A, Derosa M, Rossi M (1990) Thermostable β-galactosidase from the archaebacterium Sulfolobus solfataricus—purification and properties. Eur J Biochem 187:321–328
Rajput R, Verma VV, Chaudhary V, Gupta R (2013) A hydrolytic γ-glutamyl transpeptidase from thermoacidophilic archaeon Picrophilus torridus: binding pocket mutagenesis and transpeptidation. Extremophiles 17:29–41
Rasouli I, Kulkarni PR (1994) Enhancement of β-galactosidase productivity of Aspergillus niger NCIM-616. J Appl Bacteriol 77:359–361
Reher M, Bott M, Schonheit P (2006) Characterisation of glycerate kinase (2-phosphoglycerate forming), a key enzyme of the nonphosphorylative Entner–Doudoroff pathway, from the thermoacidophilic euryarchaeon Picrophilus torridus. FEMS Microbiol Lett 259:113–119
Saishin N, Ueta M, Wada A, Yamamoto I (2010) Properties of a β-galactosidase purified from Bifidobacterium longum subsp Longum JCM 7052 grown on gum Arabic. Int J Biol Macromol 10:23–31
Schepers B, Thiemann V, Antranikian G (2006) Characterisation of a novel glucoamylase from the thermoacidophilic Archaeon Picrophilus torridus heterologously expressed in E. coli. Eng Life Sci 6:311–317
Schleper C, Puehler G, Holz I, Gambacorta A, Janekovic D, Santarius U, Klenk HP, Zillig W (1995) Picrophilus gen. nov., fam. nov.—a novel aerobic, heterotrophic, thermoacidophilic genus and family comprising archaea capable of growth around pH 0. J Bacteriol 177:7050–7059
Sen S, Ray L, Chattopadhyay P (2012) Production, purification, immobilisation, and characterisation of a thermostable β-galactosidase from Aspergillus alliaceus. Appl Biochem Biotechnol 167:1938–1953
Serour E, Antranikian G (2002) Novel thermoactive glucoamylases from the thermoacidophilic Archaea Thermoplasma acidophilum, Picrophilus torridus and Picrophilus oshimae. Anton Leeuw Int J G 81:73–83
Shaikh SA, Khire JM, Khan MI (1999) Characterisation of a thermostable extracellular β-galactosidase from a thermophilic fungus Rhizomucor sp. Biochim Biophys Acta 1472:14–322
Sharma S, Vaid S, Bhat B, Singh S, Bajaj BK (2019) Thermostable enzymes for industrial biotechnology. Adv Enzyme Biotechnol 17:469–495
Shipkowski S, Brenchley JE (2006) Bioinformatic, genetic, and biochemical evidence that some glycoside hydrolase family 42 β-galactosidases are arabinogalactan type I oligomer hydrolases. Appl Environ Microbiol 72:7730–7738
Silvério S, Macedo EA, Teixeira JA, Rodrigues LR (2017) New β-galactosidase producers with potential for prebiotic synthesis. Bioresour Technol 250:131–139
Song YS, Lee HU, Park C, Kim SW (2012a) Batch and continuous synthesis of lactulose from whey lactose by immobilised β-galactosidase. Food Chem 136:689–694
Song YS, Shin HY, Lee JY, Park C, Kim S (2012b) β-Galactosidase-immobilised microreactor fabricated using a novel technique for enzyme immobilisation and its application for continuous synthesis of lactulose. Food Chem 133:611–617
Takagi M, Tamaki H, Miyamoto Y, Leonardi R, Hanada S, Jackowski S, Chohnan S (2010) Pantothenate kinase from the thermoacidophilic Archaeon Picrophilus torridus. J Bacteriol 192:233–241
Thurmer A, Voigt B, Angelov A, Albrecht D, Hecker M, Liebl W (2011) Proteomic analysis of the extremely thermoacidophilic archaeon Picrophilus torridus at pH and temperature values close to its growth limit. Proteomics 11:4559–4568
van Laere KM, Abee T, Schols HA, Beldman G, Voragen AG (2000) Characterisation of a novel β-galactosidase from Bifidobacterium adolescentis DSM 20083 active towards transgalacto-oligosaccharides. Appl Environ Microbiol 66:1379–1384
Wierzbicka-Wos A, Cieslinski H, Wanarska M, Kozlowska-Tylingo K, Hildebrandt P, Kur J (2011) A novel cold-active β-d-galactosidase from the Paracoccus sp. 32d-gene cloning, purification and characterisation. Microb Cell Fact 10:108
Acknowledgements
This work has been funded by the Irish Research Council under the Embark Initiative.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Moracci.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Murphy, J., Walsh, G. Purification and characterization of a novel thermophilic β-galactosidase from Picrophilus torridus of potential industrial application. Extremophiles 23, 783–792 (2019). https://doi.org/10.1007/s00792-019-01133-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-019-01133-4