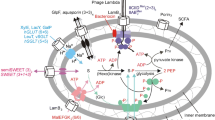
Abstract
Bacterial pathogens are a major cause of foodborne diseases and food poisoning. To cope with the acid conditions encountered in different environments such as in fermented food or in the gastric compartment, neutralophilic bacteria have developed several adaptive mechanisms. One of those mechanisms, the amino acid dependent system, consumes intracellular protons in biochemical reactions. It involves an antiporter that facilitates the exchange of external substrate amino acid for internal product and a cytoplasmic decarboxylase that catalyzes a proton-consuming decarboxylation of the substrate. So far, four acid resistance antiporters have been discovered, namely the glutamate-γ-aminobutyric acid antiporter GadC, the arginine-agmatine antiporter AdiC, the lysine-cadaverine antiporter CadB, and the ornithine-putrescine antiporter PotE. The 3D structures of AdiC and GadC, reveal an inverted-repeat fold of two times 5 transmembrane helices, typical of the amino acid-polyamine-organocation (APC) superfamily of transporters. This review summarizes our current knowledge on the transport mechanism, the pH regulation and the selectivity of these four acid resistance antiporters. It also highlights that AdiC is a paradigm for eukaryotic amino acid transporters of the APC superfamily as structural models of several of these transporters built using AdiC structures were exploited to unveil their mechanisms of amino acid recognition and translocation.







Similar content being viewed by others
References
Amano K, Ishii R, Mochizuki T, Takatsu S, Abe F (2019) Hyperactive mutation occurs adjacent to the essential glutamate 286 for transport in the yeast tryptophan permease Tat2. Biochem Biophys Res Commun 509:1047–1052. https://doi.org/10.1016/j.bbrc.2019.01.038
André B (2018) Tribute to Marcelle Grenson (1925–1996), a pioneer in the study of amino acid transport in yeast. Int J Mol Sci 19:1207. https://doi.org/10.3390/ijms19041207
Aquino P, Honda B, Jaini S, Lyubetskaya A, Hosur K, Chiu JG, Ekladious I, Hu D, Jin L, Sayeg MK, Stettner AI, Wang J, Wong BG, Wong WS, Alexander SL, Ba C, Bensussen SI, Bernstein DB, Braff D, Cha S, Cheng DI, Cho JH, Chou K, Chuang J, Gastler DE, Grasso DJ, Greifenberger JS, Guo C, Hawes AK, Israni DV, Jain SR, Kim J, Lei J, Li H, Li D, Li Q, Mancuso CP, Mao N, Masud SF, Meisel CL, Mi J, Nykyforchyn CS, Park M, Peterson HM, Ramirez AK, Reynolds DS, Rim NG, Saffie JC, Su H, Su WR, Su Y, Sun M, Thommes MM, Tu T, Varongchayakul N, Wagner TE, Weinberg BH, Yang R, Yaroslavsky A, Yoon C, Zhao Y, Zollinger AJ, Stringer AM, Foster JW, Wade J, Raman S, Broude N, Wong WW, Galagan JE (2017) Coordinated regulation of acid resistance in Escherichia coli. BMC Syst Biol 11:1–15. https://doi.org/10.1186/s12918-016-0376-y
Augustyn E, Finke K, Zur AA, Hansen L, Heeren N, Chien H-C, Lin L, Giacomini KM, Colas C, Schlessinger A, Thomas AA (2016) LAT-1 activity of meta-substituted phenylalanine and tyrosine analogs. Bioorg Med Chem Lett 26:2616–2621. https://doi.org/10.1016/j.bmcl.2016.04.023
Borsani G, Bassi MT, Sperandeo MP, De Grandi A, Buoninconti A, Riboni M, Manzoni M, Incerti B, Pepe A, Andria G, Ballabio A, Sebastio G (1999) SLC7A7, encoding a putative permease-related protein, is mutated in patients with lysinuric protein intolerance. Nat Genet 21:297–301. https://doi.org/10.1038/6815
Chang S, Hu J, Lin P, Jiao X, Tian X (2010) Substrate recognition and transport behavior analyses of amino acid antiporter with coarse-grained models. Mol BioSyst 6:2430. https://doi.org/10.1039/c005266c
Coleman JA, Green EM, Gouaux E (2016) X-ray structures and mechanism of the human serotonin transporter. Nature 532:334–339. https://doi.org/10.1038/nature17629
De Biase D, Pennacchietti E (2012) Glutamate decarboxylase-dependent acid resistance in orally acquired bacteria: function, distribution and biomedical implications of the gadBC operon. Mol Microbiol 86:770–786. https://doi.org/10.1111/mmi.12020
De Biase D, Tramonti A, Bossa F, Visca P (1999) The response to stationary-phase stress conditions in Escherichia coli: role and regulation of the glutamic acid decarboxylase system. Mol Microbiol 32:1198–1211. https://doi.org/10.1046/j.1365-2958.1999.01430.x
Deshpande AA, Sharma M, Bachhawat AK (2017) Insights into the molecular basis for substrate binding and specificity of the fungal cystine transporter CgCYN1. Biochim Biophys Acta Biomembr 1859:2259–2268. https://doi.org/10.1016/j.bbamem.2017.08.020
Diallinas G (2016) Dissection of transporter function: from genetics to structure. Trends Genet 32:576–590. https://doi.org/10.1016/j.tig.2016.06.003
Diez-Gonzalez F, Karaibrahimoglu Y (2004) Comparison of the glutamate-, arginine- and lysine-dependent acid resistance systems in Escherichia coli O157:H7. J Appl Microbiol 96:1237–1244. https://doi.org/10.1111/j.1365-2672.2004.02251.x
Errasti-Murugarren E, Fort J, Bartoccioni P, Díaz L, Pardon E, Carpena X, Espino-Guarch M, Zorzano A, Ziegler C, Steyaert J, Fernández-Recio J, Fita I, Palacín M (2019) L amino acid transporter structure and molecular bases for the asymmetry of substrate interaction. Nat Commun 10:1807. https://doi.org/10.1038/s41467-019-09837-z
Espino Guarch M, Font-Llitjós M, Murillo-Cuesta S, Errasti- Murugarren E, Celaya AM, Girotto G, Vuckovic D, Mezzavilla M, Vilches C, Bodoy S, Sahún I, González L, Prat E, Zorzano A, Dierssen M, Varela-Nieto I, Gasparini P, Palacín M, Nunes V (2018) Mutations in L-type amino acid transporter-2 support SLC7A8 as a novel gene involved in age-related hearing loss. Elife 7. https://doi.org/10.7554/eLife.31511
Fang Y, Kolmakova-Partensky L, Miller C (2007) A bacterial arginine-agmatine exchange transporter involved in extreme acid resistance. J Biol Chem 282:176–182. https://doi.org/10.1074/jbc.M610075200
Fang Y, Jayaram H, Shane T, Kolmakova-Partensky L, Wu F, Williams C, Xiong Y, Miller C (2009) Structure of a prokaryotic virtual proton pump at 3.2 Å resolution. Nature 460:1040–1043. https://doi.org/10.1038/nature08201
Feliubadaló L, Font M, Purroy J, Rousaud F, Estivill X, Nunes V, Golomb E, Centola M, Aksentijevich I, Kreiss Y, Goldman B, Pras M, Kastner DL, Pras E, Gasparini P, Bisceglia L, Beccia E, Gallucci M, de Sanctis L, Ponzone A, Rizzoni GF, Zelante L, Bassi MT, George AL Jr, Manzoni M, De Grandi A, Riboni M, Endsley JK, Ballabio A, Borsani G, Reig N, Fernández E, Estévez R, Pineda M, Torrents D, Camps M, Lloberas J, Zorzano A, Palacín M (1999) Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo, + AT) of rBAT. Nat Genet 23:52–57. https://doi.org/10.1038/12652
Forrest LR, Rudnick G (2009) The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology 24:377–386. https://doi.org/10.1152/physiol.00030.2009
Forrest LR, Krämer R, Ziegler C (2011) The structural basis of secondary active transport mechanisms. Biochim Biophys Acta 1807:167–188. https://doi.org/10.1016/j.bbabio.2010.10.014
Fotiadis D, Kanai Y, Palacín M (2013) The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34:139–158. https://doi.org/10.1016/j.mam.2012.10.007
Gao X, Lu F, Zhou L, Dang S, Sun L, Li X, Wang J, Shi Y (2009) Structure and mechanism of an amino acid antiporter. Science 324:1565–1568. https://doi.org/10.1126/science.1173654
Gao X, Zhou L, Jiao X, Lu F, Yan C, Zeng X, Wang J, Shi Y (2010) Mechanism of substrate recognition and transport by an amino acid antiporter. Nature 463:828–832. https://doi.org/10.1038/nature08741
Geier EG, Schlessinger A, Fan H, Gable JE, Irwin JJ, Sali A, Giacomini KM (2013) Structure-based ligand discovery for the large-neutral amino acid transporter 1, LAT-1. Proc Natl Acad Sci USA 110:5480–5485. https://doi.org/10.1073/pnas.1218165110
Ghaddar K, Krammer E-M, Mihajlovic N, Brohée S, André B, Prévost M (2014a) Converting the yeast arginine can1 permease to a lysine permease. J Biol Chem 289:7232–7246. https://doi.org/10.1074/jbc.M113.525915
Ghaddar K, Merhi A, Saliba E, Krammer E-M, Prévost M, André B (2014b) Substrate-induced ubiquitylation and endocytosis of yeast amino acid permeases. Mol Cell Biol 34:4447–4463. https://doi.org/10.1128/MCB.00699-14
Ghasemitarei M, Yusupov M, Razzokov J, Shokri B, Bogaerts A (2019) Transport of cystine across xC− antiporter. Arch Biochem Biophys 664:117–126. https://doi.org/10.1016/j.abb.2019.01.039
Giannella RA, Broitman SA, Zamcheck N (1972) Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut 13:251–256. https://doi.org/10.1136/gut.13.4.251
Gong S, Richard H, Foster JW (2003) YjdE (AdiC) is the arginine: agmatine antiporter essential for arginine-dependent acid resistance in Escherichia coli. J Bacteriol 185:4402–4409. https://doi.org/10.1128/JB.185.15.4402-4409.2003
Gournas C, Evangelidis T, Athanasopoulos A, Mikros E, Sophianopoulou V (2015) The Aspergillus nidulans proline permease as a model for understanding the factors determining substrate binding and specificity of fungal amino acid transporters. J Biol Chem 290:6141–6155. https://doi.org/10.1074/jbc.M114.612069
Gournas C, Prévost M, Krammer E-M, André B (2016) Function and regulation of fungal amino acid transporters: insights from predicted structure. Adv Appl Microbiol. https://doi.org/10.1007/978-3-319-25304-6_4
Gournas C, Saliba E, Krammer E-M, Barthelemy C, Prévost M, André B (2017) Transition of yeast Can1 transporter to the inward-facing state unveils an α-arrestin target sequence promoting its ubiquitylation and endocytosis. Mol Biol Cell 28:2819–2832. https://doi.org/10.1091/mbc.e17-02-0104
Gournas C, Athanasopoulos A, Sophianopoulou V (2018) On the evolution of specificity in members of the yeast amino acid transporter family as parts of specific metabolic pathways. Int J Mol Sci 19:1398. https://doi.org/10.3390/ijms19051398
Hersh BM, Farooq FT, Barstad DN, Blankenhorn DL, Slonczewski JL (1996) A glutamate-dependent acid resistance gene in Escherichia coli. J Bacteriol 178:3978–3981. https://doi.org/10.1128/jb.178.13.3978-3981.1996
Hinz KM, Meyer K, Kinne A, Schülein R, Köhrle J, Krause G (2015) Structural insights into thyroid hormone transport mechanisms of the L-type amino acid transporter 2. Mol Endocrinol 29:933–942. https://doi.org/10.1210/me.2015-1044
Hinz KM, Neef D, Rutz C, Furkert J, Köhrle J, Schülein R, Krause G (2017) Molecular features of the L-type amino acid transporter 2 determine different import and export profiles for thyroid hormones and amino acids. Mol Cell Endocrinol 443:163–174. https://doi.org/10.1016/j.mce.2017.01.024
Horák J, Ríhová L (1982) l-Proline transport in Saccharomyces cerevisiae. Biochim Biophys Acta 691:144–150
Ilgü H, Jeckelmann J-M, Gapsys V, Ucurum Z, de Groot BL, Fotiadis D (2016) Insights into the molecular basis for substrate binding and specificity of the wild-type l-arginine/agmatine antiporter AdiC. Proc Natl Acad Sci 113:10358–10363. https://doi.org/10.1073/pnas.1605442113
Ilgü H, Jeckelmann J-M, Colas C, Ucurum Z, Schlessinger A, Fotiadis D (2018) Effects of mutations and ligands on the thermostability of the l-arginine/agmatine antiporter AdiC and deduced insights into ligand-binding of human L-type amino acid transporters. Int J Mol Sci 19:918. https://doi.org/10.3390/ijms19030918
Iyer R, Williams C, Miller C (2003) arginine-agmatine antiporter in extreme acid resistance in Escherichia coli. J Bacteriol 185:6556–6561. https://doi.org/10.1128/JB.185.22.6556-6561.2003
Jack DL, Paulsen IT, Saier MH (2000) The amino acid/polyamine/organocation (APC) superfamily of transporters specific for amino acids, polyamines and organocations. Microbiology 146:1797–1814. https://doi.org/10.1099/00221287-146-8-1797
Jardetzky O (1966) Simple allosteric model for membrane pumps. Nature 211:969–970. https://doi.org/10.1038/211969a0
Kanda N, Abe F (2013) Structural and functional implications of the yeast high-affinity tryptophan permease Tat2. Biochemistry 52:4296–4307. https://doi.org/10.1021/bi4004638
Kashiwagi K, Suzuki T, Suzuki F, Furuchi T, Kobayashi H, Igarashi K (1991) Coexistence of the genes for putrescine transport protein and ornithine decarboxylase at 16 min on Escherichia coli chromosome. J Biol Chem 266:20922–20927
Kashiwagi K, Shibuya S, Tomitori H, Kuraishi A, Igarashi K (1997) Excretion and uptake of putrescine by the PotE protein in Escherichia coli. J Biol Chem 272:6318–6323. https://doi.org/10.1074/jbc.272.10.6318
Kashiwagi K, Kuraishi A, Tomitori H, Igarashi A, Nishimura K, Shirahata A, Igarashi K (2000) Identification of the putrescine recognition site on polyamine transport protein PotE. J Biol Chem 275:36007–36012. https://doi.org/10.1074/jbc.M006083200
Kashiwagi K, Miyamoto S, Suzuki F, Kobayashi H, Igarashi K (2006) Excretion of putrescine by the putrescine-ornithine antiporter encoded by the potE gene of Escherichia coli. Proc Natl Acad Sci 89:4529–4533. https://doi.org/10.1073/pnas.89.10.4529
Kowalczyk L, Ratera M, Paladino A, Bartoccioni P, Errasti-Murugarren E, Valencia E, Portella G, Bial S, Zorzano A, Fita I, Orozco M, Carpena X, Vazquez-Ibar JL, Palacín M (2011) Molecular basis of substrate-induced permeation by an amino acid antiporter. Proc Natl Acad Sci 108:3935–3940. https://doi.org/10.1073/pnas.1018081108
Krammer E-M, Ghaddar K, André B, Prévost M (2016) Unveiling the mechanism of arginine transport through AdiC with molecular dynamics simulations: the guiding role of aromatic residues. PLoS ONE 11:e0160219. https://doi.org/10.1371/journal.pone.0160219
Krammer E-M, Gibbons A, Roos G, Prévost M (2018) Molecular mechanism of substrate selectivity of the arginine-agmatine antiporter AdiC. Sci. Rep 8:15607. https://doi.org/10.1038/s41598-018-33963-1
Krause G, Hinz KM (2017) Thyroid hormone transport across L-type amino acid transporters: what can molecular modelling tell us? Mol Cell Endocrinol 458:68–75. https://doi.org/10.1016/j.mce.2017.03.018
Krishnamurthy H, Piscitelli CL, Gouaux E (2009) Unlocking the molecular secrets of sodium-coupled transporters. Nature 459:347–355. https://doi.org/10.1038/nature08143
Lin J, Lee IS, Frey J, Slonczewski JL, Foster JW (1995) Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J Bacteriol 177:4097–4104
Lu P, Ma D, Chen Y, Guo Y, Chen G-Q, Deng H, Shi Y (2013) l-Glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia. Cell Res 23:635–644. https://doi.org/10.1038/cr.2013.13
Lund P, Tramonti A, De Biase D (2014) Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev 38:1091–1125. https://doi.org/10.1111/1574-6976.12076
Ma D, Lu P, Yan C, Fan C, Yin P, Wang J, Shi Y (2012) Structure and mechanism of a glutamate—GABA antiporter. Nature 483:632–636. https://doi.org/10.1038/nature10917
Ma D, Lu P, Shi Y (2013) Substrate selectivity of the acid-activated glutamate/γ-aminobutyric acid (GABA) antiporter GadC from Escherichia coli. J Biol Chem 288:15148–15153. https://doi.org/10.1074/jbc.M113.474502
Napolitano L, Scalise M, Koyioni M, Koutentis P, Catto M, Eberini I, Parravicini C, Palazzolo L, Pisani L, Galluccio M, Console L, Carotti A, Indiveri C (2017) Potent inhibitors of human LAT1 (SLC7A5) transporter based on dithiazole and dithiazine compounds for development of anticancer drugs. Biochem Pharmacol 143:39–52. https://doi.org/10.1016/j.bcp.2017.07.006
Palazzolo L, Parravicini C, Laurenzi T, Guerrini U, Indiveri C, Gianazza E, Eberini I (2018) In silico description of LAT1 transport mechanism at an atomistic level. Front Chem 6:1–15. https://doi.org/10.3389/fchem.2018.00350
Penmatsa A, Wang KH, Gouaux E (2013) X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503:85–90. https://doi.org/10.1038/nature12533
Penmatsa A, Wang KH, Gouaux E (2015) X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat Struct Mol Biol 22:506–508. https://doi.org/10.1038/nsmb.3029
Pennacchietti E, Lammens TM, Capitani G, Franssen MCR, John RA, Bossa F, De Biase D (2009) Mutation of His 465 Alters the pH-dependent spectroscopic properties of Escherichia coli glutamate decarboxylase and broadens the range of its activity toward more alkaline pH. J Biol Chem 284:31587–31596. https://doi.org/10.1074/jbc.M109.049577
Rautio J, Gynther M, Laine K (2013) LAT1-mediated prodrug uptake: a way to breach the blood–brain barrier? Ther Deliv 4:281–284. https://doi.org/10.4155/tde.12.165
Richard H, Foster JW (2004) Escherichia coli glutamate- and arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J Bacteriol 186:6032–6041. https://doi.org/10.1128/JB.186.18.6032-6041.2004
Rolhion N, Chassaing B (2016) When pathogenic bacteria meet the intestinal microbiota. Philos Trans R Soc B Biol Sci 371:20150504. https://doi.org/10.1098/rstb.2015.0504
Rosell A, Meury M, Alvarez-Marimon E, Costa M, Perez-Cano L, Zorzano A, Fernandez-Recio J, Palacín M, Fotiadis D (2014) Structural bases for the interaction and stabilization of the human amino acid transporter LAT2 with its ancillary protein 4F2hc. Proc Natl Acad Sci 111:2966–2971. https://doi.org/10.1073/pnas.1323779111
Shaffer PL, Goehring A, Shankaranarayanan A, Gouaux E (2009) Structure and mechanism of a Na+ -independent amino acid transporter. Science 325:1010–1014. https://doi.org/10.1126/science.1176088
Shi Y (2013) Common folds and transport mechanisms of secondary active transporters. Annu Rev Biophys 42:51–72. https://doi.org/10.1146/annurev-biophys-083012-130429
Singh N, Ecker GF (2018) Insights into the structure, function, and ligand discovery of the large neutral amino acid transporter 1, lat1. Int J Mol Sci 19:1–32. https://doi.org/10.3390/ijms19051278
Soksawatmaekhin W, Kuraishi A, Sakata K, Kashiwagi K, Igarashi K (2004) Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol Microbiol 51:1401–1412. https://doi.org/10.1046/j.1365-2958.2003.03913.x
Soksawatmaekhin W, Uemura T, Fukiwake N, Kashiwagi K, Igarashi K (2006) Identification of the cadaverine recognition site on the cadaverine-lysine antiporter CadB. J Biol Chem 281:29213–29220. https://doi.org/10.1074/jbc.M600754200
Sophianopoulou V, Scazzocchio C (1989) The proline transport protein of Aspergillus nidulans is very similar to amino acid transporters of Saccharomyces cerevisiae. Mol Microbiol 3:705–714. https://doi.org/10.1111/j.1365-2958.1989.tb00219.x
Tărlungeanu DC, Deliu E, Dotter CP, Kara M, Janiesch PC, Scalise M, Galluccio M, Tesulov M, Morelli E, Sonmez FM, Bilguvar K, Ohgaki R, Kanai Y, Johansen A, Esharif S, Ben-Omran T, Topcu M, Schlessinger A, Indiveri C, Duncan KE, Caglayan AO, Gunel M, Gleeson JG, Novarino G (2016) Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder. Cell 167:1481-1494.e18. https://doi.org/10.1016/j.cell.2016.11.013
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Torrents D, Mykkänen J, Pineda M, Feliubadaló L, Estévez R, de Cid R, Sanjurjo P, Zorzano A, Nunes V, Huoponen K, Reinikainen A, Simell O, Savontaus M-L, Aula P, Palacín M (1999) Identification of SLC7A7, encoding y+LAT-1, as the lysinuric protein intolerance gene. Nat Genet 21:293–296. https://doi.org/10.1038/6809
Tsai M-F, Miller C (2013) Substrate selectivity in arginine-dependent acid resistance in enteric bacteria. Proc Natl Acad Sci 110:5893–5897. https://doi.org/10.1073/pnas.1301442110
Tsai M-F, Fang Y, Miller C (2012) Sided functions of an arginine-agmatine antiporter oriented in liposomes. Biochemistry 51:1577–1585. https://doi.org/10.1021/bi201897t
Tsai M-F, McCarthy P, Miller C (2013) Substrate selectivity in glutamate-dependent acid resistance in enteric bacteria. Proc Natl Acad Sci 110:5898–5902. https://doi.org/10.1073/pnas.1301444110
Usami Y, Uemura S, Mochizuki T, Morita A, Shishido F, Inokuchi J, Abe F (2014) Functional mapping and implications of substrate specificity of the yeast high-affinity leucine permease Bap2. Biochim Biophys Acta 1838:1719–1729. https://doi.org/10.1016/j.bbamem.2014.03.018
Wang S, Yan R, Zhang X, Chu Q, Shi Y (2014) Molecular mechanism of pH-dependent substrate transport by an arginine-agmatine antiporter. Proc Natl Acad Sci 111:12734–12739. https://doi.org/10.1073/pnas.1414093111
Wang KH, Penmatsa A, Gouaux E (2015) Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 521:322–327. https://doi.org/10.1038/nature14431
Wong FH, Chen JS, Reddy V, Day JL, Shlykov MA, Wakabayashi ST, Saier MH Jr (2012) The amino acid-polyamine-organocation superfamily. J Mol Microbiol Biotechnol 22:105–113. https://doi.org/10.1159/000338542
Yan R, Zhao X, Lei J, Zhou Q (2019) Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature. https://doi.org/10.1038/s41586-019-1011-z
Zomot E, Bahar I (2011) Protonation of glutamate 208 induces the release of agmatine in an outward-facing conformation of an arginine/agmatine antiporter. J Biol Chem 286:19693–19701. https://doi.org/10.1074/jbc.M110.202085
Acknowledgements
M.P. is a senior research associate and E.M.K. is a postdoctoral researcher of the Fonds de la Recherche Scientifique de Belgique (F.R.S.-F.R.N.S.), Belgium. This work was supported by an ARC Grant (AUWB 2010-15-2) from the Fédération Wallonie-Bruxelles and by the Fonds National de La Recherche Scientifique (PDR T.1107.15).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krammer, EM., Prévost, M. Function and Regulation of Acid Resistance Antiporters. J Membrane Biol 252, 465–481 (2019). https://doi.org/10.1007/s00232-019-00073-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-019-00073-6