Abstract
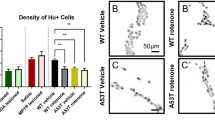
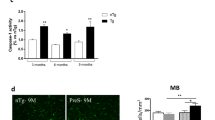
Emerging findings suggest that Parkinson’s disease (PD) pathology (α-synuclein accumulation) and neuronal dysfunction may occur first in peripheral neurons of the autonomic nervous system including the enteric branches of the vagus nerve. The risk of PD increases greatly in people over the age of 65, a period of life in which chronic inflammation is common in many organ systems including the gut. Here we report that chronic mild focal intestinal inflammation accelerates the age of disease onset in α-synuclein mutant PD mice. Wild-type and PD mice treated with 0.5% dextran sodium sulfate (DSS) in their drinking water for 12 weeks beginning at 3 months of age exhibited histological and biochemical features of mild gut inflammation. The age of onset of motor dysfunction, evaluated using a rotarod test, gait analysis, and grip strength measurements, was significantly earlier in DSS-treated PD mice compared to control PD mice. Levels of the dopaminergic neuron marker tyrosine hydroxylase in the striatum and numbers of dopaminergic neurons in the substantia nigra were reduced in PD mice with gut inflammation. Levels of total and phosphorylated α-synuclein were elevated in enteric and brain neurons in DSS-treated PD mice, suggesting that mild gut inflammation accelerates α-synuclein pathology. Markers of inflammation in the colon and brain, but not in the blood, were elevated in DSS-treated PD mice, consistent with retrograde transneuronal propagation of α-synuclein pathology and neuroinflammation from the gut to the brain. Our findings suggest that interventions that reduce gut inflammation may prove beneficial in the prevention and treatment of PD.







Similar content being viewed by others
References
Adams-Carr, K. L., Bestwick, J. P., Shribman, S., Lees, A., Schrag, A., & Noyce, A. J. (2016). Constipation preceding Parkinson’s disease: A systematic review and meta-analysis. Journal of Neurology, Neurosurgery and Psychiatry, 87, 710–716.
Allen Reish, H. E., & Standaert, D. G. (2015). Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. Journal of Parkinson’s Disease, 5, 1–19.
Awerbuch, G. I., & Sandyk, R. (1994). Autonomic functions in the early stages of Parkinson’s disease. International Journal of Neuroscience, 74, 9–16.
Bencsik, A., Muselli, L., Leboidre, M., Lakhdar, L., & Baron, T. (2014). Early and persistent expression of phosphorylated α-synuclein in the enteric nervous system of A53T mutant human α-synuclein transgenic mice. Journal of Neuropathology and Experimental Neurology, 73, 1144–1151.
Chandra, S., Gallardo, G., Fernández-Chacón, R., Schlüter, O. M., & Südhof, T. C. (2005). Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell, 123, 383–396.
Choi, D. Y., Liu, M., Hunter, R. L., Cass, W. A., Pandya, J. D., Sullivan, P. G., et al. (2009). Striatal neuroinflammation promotes Parkinsonism in rats. PLoS ONE, 4(5), e5482.
Chung, C. Y., Koprich, J. B., Siddiqi, H., & Isacson, O. (2009). Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. Journal of Neuroscience, 29, 3365–3373.
Dawson, T. M., Ko, H. S., & Dawson, V. L. (2010). Genetic animal models of Parkinson’s disease. Neuron, 66, 646–661.
Del Tredici, K., & Braak, H. (2016). Review: Sporadic Parkinson’s disease: Development and distribution of α-synuclein pathology. Neuropathology and Applied Neurobiology, 42, 33–50.
Del Tredici, K., Rüb, U., De Vos, R. A., Bohl, J. R., & Braak, H. (2002). Where does parkinson disease pathology begin in the brain? Journal of Neuropathology and Experimental Neurology, 61, 413–426.
Devos, D., Lebouvier, T., Lardeux, B., Biraud, M., Rouaud, T., Pouclet, H., et al. (2013). Colonic inflammation in Parkinson’s disease. Neurobiology of Diseases, 50, 42–48.
Dias, V., Junn, E., & Mouradian, M. M. (2013). The role of oxidative stress in Parkinson’s disease. Journal of Parkinson’s Disease, 3, 461–491.
Farrand, A. Q., Helke, K. L., Gregory, R. A., Gooz, M., Hinson, V. K., & Boger, H. A. (2017). Vagus nerve stimulation improves locomotion and neuronal populations in a model of Parkinson’s disease. Brain Stimulation, 10, 1045–1054.
Gao, H. M., Zhang, F., Zhou, H., Kam, W., Wilson, B., & Hong, J. S. (2011). Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environmental Health Perspectives, 119, 807–814.
Gazewood, J. D., Richards, D. R., & Clebak, K. (2013). Parkinson disease: An update. American Family Physician, 87, 267–273.
Griffioen, K. J., Rothman, S. M., Ladenheim, B., Wan, R., Vranis, N., Hutchison, E., et al. (2013). Dietary energy intake modifies brainstem autonomic dysfunction caused by mutant α-synuclein. Neurobiology of Aging, 34, 928–935.
Hall, B., Mak, E., Cervenka, S., Aigbirhio, F. I., Rowe, J. B., & O’Brien, J. T. (2017). In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings. Ageing Research Reviews, 36, 50–63.
Hallett, P. J., McLean, J. R., Kartunen, A., Langston, J. W., & Isacson, O. (2012). α-Synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiology of Diseases, 47, 258–267.
Hirsch, E. C., Vyas, S., & Hunot, S. (2012). Neuroinflammation in Parkinson’s disease. Parkinsonism and Related Disorders, 18(Suppl 1), S210–S212.
Holmqvist, S., Chutna, O., Bousset, L., Aldrin-Kirk, P., Li, W., Björklund, T., et al. (2014). Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathologica, 128, 805–820.
Houlden, H., & Singleton, A. B. (2012). The genetics and neuropathology of Parkinson’s disease. Acta Neuropathologica, 124, 325–338.
Klingelhoefer, L., & Reichmann, H. (2015). Pathogenesis of Parkinson disease–the gut-brain axis and environmental factors. Nature Reviews Neurology, 11, 625–636.
Lai, S. W., Liao, K. F., Lin, C. L., & Sung, F. C. (2014). Irritable bowel syndrome correlates with increased risk of Parkinson’sdisease in Taiwan. European Journal of Epidemiology, 29, 57–62.
Liu, B., Fang, F., Pedersen, N. L., Tillander, A., Ludvigsson, J. F., Ekbom, A., et al. (2017). Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology, 88, 1996–2002.
Mattson, M. P. (2012). Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metabolism, 16, 706–722.
Mattson, M. P., & Wan, R. (2005). Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. Journal of Nutritional Biochemistry, 16, 129–137.
Murphy, S. L., Xu, J., & Kochanek, K. D. (2012). Deaths: Preliminary data for 2010. CDC National Vital Statistics Reports, 60, 1–52.
Noorian, A. R., Rha, J., Annerino, D. M., Bernhard, D., Taylor, G. M., & Greene, J. G. (2012). Alpha-synuclein transgenic mice display age-related slowing of gastrointestinal motility associated with transgene expression in the vagal system. Neurobiology of Diseases, 48, 9–19.
Oueslati, A. (2016). Implication of alpha-synuclein phosphorylation at S129 in synucleinopathies: What have we learned in the last decade? Journal of Parkinson’s Disease, 6, 39–51.
Pan-Montojo, F., Schwarz, M., Winkler, C., Arnhold, M., O’Sullivan, G. A., Pal, A., et al. (2012). Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Scientific Reports, 2, 898.
Phillips, R. J., Walter, G. C., Wilder, S. L., Baronowsky, E. A., & Powley, T. L. (2008). Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: Autonomic pathway implicated in Parkinson’sdisease? Neuroscience, 153, 733–750.
Pickrell, A. M., & Youle, R. J. (2015). The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron, 85, 257–273.
Prigent, A., Gonzales, J., Durand, T., Le Berre-Scoul, C., Rolli-Derkinderen, M., Neunlist, M., et al. (2018). Acute inflammation down-regulates alpha-synuclein expression in enteric neurons. Journal of Neurochemistry, 148(6), 746–760. https://doi.org/10.1111/jnc.14656.
Reichardt, F., Chassaing, B., Nezami, B. G., Li, G., Tabatabavakili, S., Mwangi, S., et al. (2017). Western diet induces colonic nitrergic myenteric neuropathy and dysmotility in mice via saturated fatty acid- and lipopolysaccharide-induced TLR4 signalling. Journal of Physiology, 595, 1831–1846.
Rotermund, C., Truckenmüller, F. M., Schell, H., & Kahle, P. J. (2014). Diet-induced obesity accelerates the onset of terminal phenotypes in α-synuclein transgenic mice. Journal of Neurochemistry, 131, 848–858.
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell, 167, 1469–1480.
Savica, R., Rocca, W. A., & Ahlskog, J. E. (2010). When does Parkinson disease start? Archives of Neurology, 67, 798–801.
Sconce, M. D., Churchill, M. J., Greene, R. E., & Meshul, C. K. (2015). Intervention with exercise restores motor deficits but not nigrostriatal loss in a progressive MPTP mouse model of Parkinson’s disease. Neuroscience, 299, 156–174.
Stokholm, M. G., Danielsen, E. H., Hamilton-Dutoit, S. J., & Borghammer, P. (2016). Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Annals of Neurology, 79, 940–949.
Svensson, E., Horváth-Puhó, E., Thomsen, R. W., Djurhuus, J. C., Pedersen, L., Borghammer, P., et al. (2015). Vagotomy and subsequent risk of Parkinson’s disease. Annals of Neurology, 78, 522–529.
Tansey, M. G., Frank-Cannon, T. C., McCoy, M. K., Lee, J. K., Martinez, T. N., McAlpine, F. E., et al. (2008). Neuroinflammation in Parkinson’s disease: Is there sufficient evidence for mechanism-based interventional therapy? Front Biosci, 13, 709–717.
Travagli, R. A., & Anselmi, L. (2016). Vagal neurocircuitry and its influence on gastric motility. Nature Reviews Gastroenterology and Hepatology, 13, 389–401.
Ulusoy, A., Rusconi, R., Pérez-Revuelta, B. I., Musgrove, R. E., Helwig, M., Winzen-Reichert, B., et al. (2013). Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Molecular Medicine, 5, 1119–1127.
Villemagne, V. L. (2016). Amyloid imaging: Past, present and future perspectives. Ageing Research Reviews, 30, 95–106.
Villumsen, M., Aznar, S., Pakkenberg, B., Jess, T., & Brudek, T. (2018). Inflammatory bowel disease increases the risk of Parkinson’s disease: A Danish nationwide cohort study 1977-2014. Gut, 68(1), 18–24.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kishimoto, Y., Zhu, W., Hosoda, W. et al. Chronic Mild Gut Inflammation Accelerates Brain Neuropathology and Motor Dysfunction in α-Synuclein Mutant Mice. Neuromol Med 21, 239–249 (2019). https://doi.org/10.1007/s12017-019-08539-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-019-08539-5